The pharmaceutical industry invests a significant amount of time and money every year writing and updating Standard Operating Procedures (SOPs).
But it doesn’t appear to be having an impact on the number of 483’s issued by the FDA…
In fact, in Biologics manufacturing, issues with SOPs are the number one reason for companies being issued a 483.
While the number had been trending downwards over recent years, 2017 actually showed a 35% increase compared to 2016…
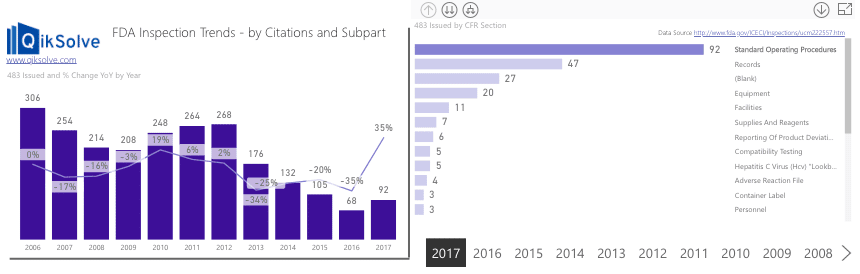
Current investment in new/updated SOPs is not yet delivering the necessary return on investment to the industry.
But why?
An SOP, by definition, is laying out the necessary steps or a procedure. Why are teams finding it so hard to implement? Why are some procedures seemingly so hard to comprehend?
The Ever-Expanding Procedure
Even with the best of intentions a new method can be developed, translated into a procedure, implemented and then seemingly perpetually updated as time goes by.
Regular updates are often the result of the ‘exceptions that make the rule’.
In industry, timelines are always a factor. There is rarely enough time to be perfect or to conduct extensive analytics. As a result, procedures and updates are sometimes rushed through, resulting in unintended consequences and misinterpretations.
Once everyone is trained, and as soon as a procedure is implemented, deviations and observations can come flooding in.
So how can you know you’ve got a procedure right?
In work, just as in life, you often need to be looking for the obvious evidence and not-so-obvious signs that you have taken the right path.
But the good news is, there are a few things you can do up-front to give you the best possible chance of getting it right first time.
Here are 6 tips to writing more robust SOPs. Following these steps should save you money and ultimately, reduce deviations.
Tip 1 – Think like a trainer…
When preparing a procedure, think like a trainer. Consider:
- Who is the intended audience?
- What are the common and the unique learning outcomes for the disciplines involved?
- How will you measure those outcomes?
And, perhaps most importantly, what are the signs to look out for that will suggest you are unintentionally sliding away from the procedure’s original intent?
Knowing these at the outset means you’re much more likely to spot them if they happen.
Don’t let exceptions detract from the intent.
Don’t allow the procedure to become oppressive to the those who have to implement it.
If it is faulty, fix it rather than defending it.
Tip 2 – … then think like a risk manager
Get all stakeholders involved at the outset and clearly define the procedure’s intent. Doing so means you won’t be relentlessly trying to force a procedure that’s not workable.
Get their opinion on the practicalities, on the impact on product quality and safety, and on the business itself. Make sure you have the right people (both internal and external) with the right expertise to take part in this exercise.
Risk assess the new procedure and test it robustly via simulation (or otherwise) prior to implementation.
Ask yourself in advance “What unforeseen actions would give undesirable outcomes for the product, processes or the business?”.
Mitigate as many of these risks as possible, early on.
Tip 3 – Talk to the training department
Work with the training department to clearly define learning aims and outcomes.
Once you have this, design an effective training program with clear and reliable assessments that are measurable.
One procedure can lead to many training packages as you tailor them to talk to distinct or combined groups from across several disciplines.
Tip 4 – Time is your best friend
When you’re developing a procedure, allocate the time that’s practically allowable by the business… and then add a little more! It will usually be worth it in the long run.
Tip 5 – Find the right words
Understanding something and writing it so others can understand it, are two very different things.
If you find yourself having to explain and ‘defend’ your document multiple times, it probably isn’t as clear as you would have liked.
When you’re writing, be sure to consider:
- How familiar is the intended audience with the language of the organisation?
- Are they new or legacy employees?
Keep in mind that organisation-specific terminology that is different from (and possibly conflicting with) standard industry terminology, should not be included in the procedure.
Tip 6 – Don’t lose sight of the primary goal
Where exceptions drive procedure updates, the content can sometimes wrongly be allowed to expand too much.
Similarly, as a procedure is updated, the accompanying training can become too focused on these updates.
If these things are allowed to happen, eventually the exceptions and singular focus on updates begin to detract from the overall purpose of the procedure.
Basic understanding of the procedure’s intent can be lost. The original well-meaning message can be drowned out.
Don’t let this happen to your critical procedures.
Save money; reduce deviations
All companies put time, effort, and significant resources into creating SOPs. No matter how good the initial intentions, it can be easy to make simple mistakes that, in the long run, can undermine your goal.
By following the steps above, you should be well-equipped to predict problems before they arise and work with the wider team to create and maintain SOPs that are truly fit for purpose.
If you can accomplish that, your company will be in a great position to save money with SOPs that stand the test of time (and avoid SOP-related 483s!).
Good luck!
About the Author
Dr. Joe Brady
Full-Time Validation Lead
Lecturer, Technological University Dublin, Ireland
Senior Associate, GetReskilled
Dr. Joe Brady is a full-time validation lead and also lectures with Technological University Dublin (TU Dublin), in the School of Chemical and Pharmaceutical Sciences. Joe is a certified trainer and highly experienced in competency-based training. He designs and prepares educational modules and full academic courses ranging from MSc, MEngSc. BSc, to Certificate level, for a range of academic institutions.
He is also a supervisor for MSc/MEngSc and Ph.D. theses. Joe has over twenty years of project experience in the pharmaceutical, biopharmaceutical, and medical device industries in Ireland, Singapore, China, The Netherlands, France and the USA.
Gerry Creaner
President
Senior Lecturer with GetReskilled
Gerry Creaner has over 40-years of experience in the Life Sciences Manufacturing industry across a range of technical, managerial and business roles. He established a very successful engineering consultancy prior to founding GetReskilled, an online education and learning business, with offices in Singapore, Ireland and Boston (USA), focussed on the manufacture of safe and effective medicines for the public.
He is also a founding Director of two Singapore based philanthropic organizations, the Farmleigh Fellowship and the Singapore-Ireland Fund, both of which deepen the well established and historical Singapore – Ireland relationship and deliver long-term benefits to both countries.
Gerry has an undergraduate degree in Chemical Engineering (UCD, 1980) and an MSc (Management) from Trinity College Dublin (2003) and is currently doing research for his Ph.D.


Post Your Comments Below