What is a Critical Components List Used For In Pharma? How do you Use Impact Assessments to Build an Effective List?
By: Gerry Creaner B.Chem Eng and Donagh Fitzgerald B.Prod Eng. Last Updated: July 2024

Image: Genengnews
A critical components list is a list of equipment, ancillary equipment, instruments and piping elements that directly or indirectly impact product quality and patient safety. The items on the list will be the focus of our qualification efforts when building a new or modifying an existing plant or facility and will be focused on by the regulatory authorities (FDA, EMA, etc) when they come to approve the medicines that are being manufactured in that facility.
This component list will form the foundation for our “Equipment Qualification Protocol” which is a written plan stating how equipment qualification will be conducted. The protocol lists factors such as:
- product characteristics
- production equipment
- test scripts and methods
- test parameters
- acceptance criteria
- test checksheets
- final approval
The objective of a protocol is to prove “fitness for use”( i.e. the system works) of the critical components within an equipment system.
BTW, if you need to learn how to develop an IQ IQ PQ equipment qualification protocol, check out our Equipment Validation (IQ OQ PQ) Training Course – For Starter Validation, CQV and C&Q Roles.
Why do we need a critical components list?
Imagine you have to qualify a new manufacturing facility.
Let’s think about this – your factory is made up of numerous different systems such as:
- Parking facilities
- Elevators
- Office air conditioner
- Chilled water production
- Air conditioner
- Building management system
- Autoclave
- Purified water system
- Clean-In-Place (CIP) system
Each one of these systems is made up of lots of different items, components, subsystems and instruments.
There can be a tendency, especially amongst novice CQV, C&Q, validation technicians and engineers to err on the side of caution and qualify 100% of the components in every system. After all, surely that’s the safest approach.
But as the economist Thomas Sowell said;
“There are no solutions, there are only trade-offs”
And there are certainly some huge trade-offs with a “qualifying 100% of items in every system” approach.
For example:
1) It’s hugely expensive and wasteful
Some of the systems and the items within them will have a disproportionately large impact on product quality, while other systems and items will have none at all. Qualifying items that don’t directly impact product quality or patient safety simply wastes time and resources. It makes far more sense to focus your attention and resources on the crucial systems and items.
2) It makes medicines less affordable
Ultimately your goal is to make safe medicines at an affordable cost. The qualification process, while necessary, is enormously time-consuming and document-heavy. Qualifying 100% of items would drive the cost of the project and the subsequent final price of the medicine way higher than necessary.
3) It increases the regulatory burden and cost of compliance
All items on the critical components list will be focused on by the regulatory authorities (FDA, EMA, etc) when they come to approve the medicines that are being manufactured in that facility. So every single item you add to the list increases the regulatory burden and cost of compliance.
4) Qualification is not a one-off cost
There is a huge operational cost with qualification. Every item on the list becomes strictly controlled and any subsequent change or modification to the item must be documented and managed as per the change control procedure. Details might include a description of the current situation, an outline of the change, justification for the change, an impact assessment based on the areas it affects, a risk assessment, an action plan and a final evaluation of the change (European Medicines Agency 2007, p.13).
5) It increases the risk of something being missed
This is arguably the biggest trade-off of all.
You may have heard the expression,
“If everything is important, then nothing is.”
If you place all your attention equally on all systems and all items within every system, you increase the chances of something critical being missed which could in turn harm the patient. Not every system or item has a critical impact on product quality and patient safety and, from a risk assessment perspective, it makes no sense to treat all equipment items as if they do.
The Solution? Conduct Impact Assessments!
So if 100% qualification is not the answer, what is?
The solution is to conduct impact assessments.
This is a procedure where the effects of different systems (and the components of each system) on product quality and patient safety are assessed, to uncover the most critical items. You then focus your qualification efforts solely on those critical items.
Impact assessments are conducted on two levels.
STAGE 1 – Conduct a system-level impact assessment to figure out which systems within the plant are crucial. This process will help you determine what systems have:
- Direct Impact – has a direct impact on product quality
- Indirect Impact – doesn’t have a direct impact on product quality but supports a system that does
- No Impact
Our qualification efforts will only focus on those systems with “Direct Impact” and “Indirect Impact”.
STAGE 2 – Conduct a component-level impact assessment within those systems identified in STAGE 1, to figure out which components within those critical systems are crucial to product quality. This process will help you determine what components are:
- Product Contact Critical – the item has direct or indirect contact with the product
- Operationally Critical – the item is crucial to the correct operation of the process
- Non Critical
Our qualification efforts will only focus on those components that are deemed “Product Contact Critical” and “Operationally Critical” within “Direct Impact” and “Indirect Impact” systems.
Typically, the system-level impact assessments and component-level impact assessments are performed during the preparation of the protocol.
Based on this assessment, the critical elements are determined and these are included within the scope of the equipment qualification protocol. This helps strike a balance between product quality and patient safety and excessive cost.
Let’s take a look at these steps in more detail.
System-Level Impact Assessment
We conduct a system-level impact assessment to figure out which systems within the plant are crucial using the following steps.
1) Determining System Boundaries
First, using the ISPE Baseline Guide Volume-5 “Commissioning and Qualification”, you determine the system boundaries and define what is, and is not, included in the system. You need to have an understanding of system functionality in order to define a system.
2) Evaluate the impact
Once boundaries are logically defined, you apply the guide to evaluate systems as:
- Direct Impact – where the system “is expected to have a direct impact on product quality”
- Indirect Impact – where the “system is not expected to have a direct impact on product quality, but typically will support a Direct Impact System”
- No Impact – where the system “will not have any impact, either directly or indirectly, on product quality”
A system is generally determined to be of direct or indirect impact if it:
- Affects the product’s safety, integrity, strength, purity, or quality
- Produces/delivers a gas or liquid that has direct contact with the product or is used as an ingredient in the product
- Is used in cleaning, sanitizing, and sterilizing direct product contact surfaces of process equipment (e.g. clean steam)
- Preserves product status (e.g. environmental control systems, nitrogen purge for oxygen-sensitive products)
- Produces data, which is used to make quality decisions (accept/reject) regarding product status (e.g. electronic batch record system, critical process parameter chart recorder)
An example of a completed system impact assessment might look something like this:
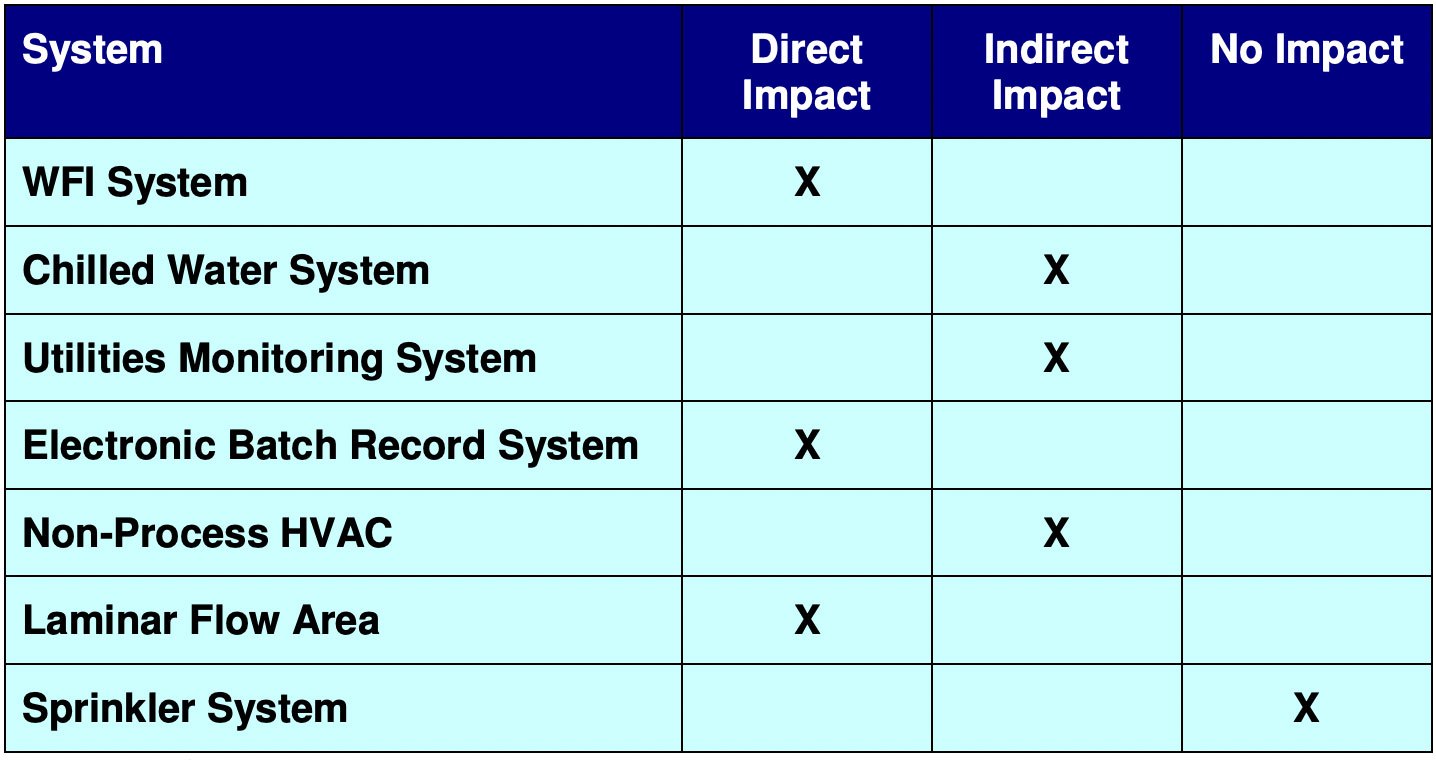
The Impact Spectrum
On the chart below, we see that some of these systems like the “purified water system” have a direct impact on patient safety. Others like the “office air conditioner” have none. But the distinction between “direct impact”, “indirect impact” or “no impact” is not always clear-cut.
Rather the system impact falls on a spectrum. And this is where judgement and experience come into play to make the distinction between types of impact.
We use Good Engineering Practices (GEPs) to manage the design, installation and commissioning of systems and their components within our facility. GEPs have been developed over decades of trial and error and hard-won experience and are a set of industry practices used to manage engineering projects.
In addition to GEPs, we must also qualify all systems that have a “direct impact” or “indirect impact” on product quality and patient safety.
The regulatory authorities (FDA, EMA, etc) will focus on these qualified systems when they come to approve the medicines that are being manufactured in that facility.

Component-Level Impact Assessment
A component-level impact assessment evaluates the criticality of the components within the system by considering their impact on product quality.
You conduct this assessment on the items within systems you’ve identified as critical, to figure out what specific components are critical to product quality and patient safety. The ultimate goal will be to only qualify the items that are critical within the system.
We apply the following criteria to each item within the system to determine its criticality.
Product Contact Critical
A component is listed as “Product Contact Critical” if it has:
- Direct contact with product or product components
Or
- Is in contact with a material that will contact the product downstream
Product-contact critical items come into direct contact with the product OR are part of the chain of surfaces through which there is a risk of contamination being carried to the product.
The first part of that is quite self-explanatory but the second part can take a bit of thinking about. So let’s consider an everyday example…
You’re in the kitchen and you cut raw chicken. Once you’ve finished, you don’t wash your hands. Instead, you go to the fridge and take out salad items. You cut them, and serve the salad to your friend. Your friend gets ill from salmonella after eating the salad.
Now your friend was never near the raw chicken but there was a chain of surfaces (i.e. the skin on your hands and the outer surface of the salad items) that brought the salmonella directly from the raw chicken to your friend.
Let’s look at the example of a cleaning system in a pharmaceutical plant for interior surfaces of pipes, pumps, tanks vessels, equipment, filters, etc.

Clean-In-Place (CIP) System – Image: Lonza
These systems are known as Clean-In-Place (CIP) systems. See the photo above.
And while these systems are not used for manufacturing, they are used to clean the manufacturing systems between production batches and contain many product-contact critical items.
The final medicine or its ingredients never come near the Clean-In-Place (CIP) system but the system is part of a chain of surfaces that can pass contamination from the product to the cleaning systems and back to the product again.
Take any of the tanks in the photo of the Clean-In-Place system above. The inner surface of the tank touches the detergent liquid. That detergent liquid passes along a system of pipes and into the reactors (not shown in the photo) during the cleaning operation.
That same liquid then touches the inner surface of the reactor. After a cleaning cycle is finished and manufacturing restarts, the inside of that reactor will come into direct contact with product ingredients.
So although no final products (or even ingredients) are ever in direct contact with the inside of the detergent tank, there is a chain of surfaces that leads from the tank to a surface that will come into contact with the product.
If there was contamination in the detergent tank, there is a possibility it could ultimately reach the product via this chain of surfaces. Therefore, our detergent tank is product-contact critical.
Operationally Critical
Operationally critical items don’t come into direct contact with the product and they’re not part of that chain of surfaces that can introduce contamination.
But they contribute to maintaining a state of balance (dynamic equilibrium) for the key factors (i.e. temperature, flow, level, concentration) and are critical to the operation of the system.
If an operationally critical component/instrument fails, product quality is immediately affected.
If a component matches any of the 6 criteria taken from our qualification course, it is deemed “Operationally Critical”:
- The component is used to demonstrate compliance with a process
- The normal operation or control of the component has a direct effect on product quality
- Failure or alarm of the component will have a direct effect on product quality or efficacy
- Information from this component is recorded as part of the batch record, lot release data, or other GMP-related documentation
- The component controls critical process elements that may affect product quality, without independent verification of the control system performance
- The component is used to create or preserve a critical status of a system
Non-critical
Non-critical items do not come into contact with the product, nor can they influence the factors that are essential for the correct functioning of the system.
Documenting a component-level impact assessment
Let’s look at the example of a Clean-In-Place (CIP) system taken from our training course on equipment validation. All the items within the system are itemized across the following four lists.
- Equipment list
- Instrument list
- Piping list
- Inline component list
Normally we would take the relevant items from each list and add them to a spreadsheet with the following layout. In this example, we have taken components from the equipment list and added them to the table below.

You then work step-by-step through the list to determine the criticality of each component.
First, we ask if it is product contact critical.
Then we ask if it is Operational critical.
If it is neither, then you deem the component “Non Critical”
Advice to Those New To Conducting Impact Assessments
The key thing throughout all of this is to remember:
- Impact Assessment is an informed judgment, made by a group of appropriately qualified stakeholders, and should be based on a comprehensive understanding of the product, process, and nature of the systems and components. You need to be able to justify your decisions and explicitly state and document a well-thought-out rationale in a concise manner.
- There is more than one right answer to each and every question. You will regularly come across situations where the criticality can be argued two different ways for the same piece of equipment or instrument. There is no such thing as “only one right answer”. In fact, there are multiple right answers and approaches. The key point is that you must be able to explain your rationale to your supervisor or an FDA or EMA auditor. As long as your rationale is logically sound, where someone who disagrees with you can understand your decision, you won’t be penalised (even if you are asked to change it).
- Have confidence in your decision and be confident that you can explain your rationale to a supervisor or an FDA auditor. Even if someone disagrees with you, so long as they can follow your logic and reasoning, you won’t be in trouble.
- In a real-world setting, especially at the beginning of a pharmaceutical validation career, you’ll be working within a larger team. You’ll be given specific tasks and responsibilities by a senior validation team member and it’s very unlikely that you’d ever have to complete an impact assessment by yourself.
Equipment Qualification Training Course
If you need to learn how to develop an IQ IQ PQ equipment qualification protocol, check out our Equipment Validation (IQ OQ PQ) Training Course – For Starter Validation, CQV and C&Q Roles.
Next Steps
About the Author
Gerry Creaner
President
Senior Lecturer with GetReskilled
Gerry Creaner has over 40-years of experience in the Life Sciences Manufacturing industry across a range of technical, managerial and business roles. He established a very successful engineering consultancy prior to founding GetReskilled, an online education and learning business, with offices in Singapore, Ireland and Boston (USA), focussed on the manufacture of safe and effective medicines for the public.
He is also a founding Director of two Singapore based philanthropic organizations, the Farmleigh Fellowship and the Singapore-Ireland Fund, both of which deepen the well established and historical Singapore – Ireland relationship and deliver long-term benefits to both countries.
Gerry has an undergraduate degree in Chemical Engineering (UCD, 1980) and an MSc (Management) from Trinity College Dublin (2003) and is currently doing research for his Ph.D.
Donagh Fitzgerald
Head of Marketing & Product Development
Mechanical/Production Engineer
Donagh looks after the marketing and product development including the training and pedagogical elements of our programs and makes sure that all GetReskilled’s users can have a great online learning experience. Donagh has lived and worked in many countries including Ireland, America, the UK, Singapore, Hong Kong and Japan. Donagh has also served as the Program Manager for the Farmleigh Fellowship based out of Singapore.
Donagh holds Degrees in Production Engineering and Mechanical Engineering from South East Technological University, Ireland.

