Your Pathway to a Career in Validation
By: Claire Wilson BSc. Last Updated: Jan 2023

Validation is a specialist career path within pharmaceutical and medical device manufacturing. The purpose of this team is to create documentation and run tests to show that a manufacturing process and the equipment associated with it will produce a consistent and reliable result.
(We’ve got a much more detailed article on validation in pharmaceutical manufacturing industry if that’s what you’re looking for).
Validation roles are best suited to certain types of people
The most successful members of validation teams generally have the following:
- Strong documentation bias – as you will be managing and documenting the entire validation process
- Enthusiasm for working in a team – validation of equipment systems is a team activity and you will need good verbal and listening skills to share information amongst the team members
- Good computer skills – there is a lot of documentation processed via computer systems.
- Structured approach to working – your work will impact others in the overall Validation team, there will be many documents that require sharing and signing with multiple team members
(Be sure to check out the 8 most “in demand” skills for validation professionals for much more detail on this.)
Job roles within the team can vary
(for those with links, you can click to get a detailed look at what the role involves)
- Validation Technician – Some pharma companies call this role:
- Associate QA Validation Specialist
- Equipment Validation Specialist
- Equipment & Process Validation Specialist
- QA Validation Associate
- QA Validation Specialist
- Senior Validation Specialist
- CQV Specialist – Some pharma companies call this role:
- CQV Technician
- C&Q Specialist
- C&Q Junior Project Manager
With validation, CQV and C&Q roles, you could work directly for:
- pharmaceutical companies on in-house projects
- engineering consultancies as part of a project team on small to large capital projects
- engineering contractors as part of a project team on small to large capital projects
And with more experience:
- Validation Engineer – a more senior member of validation teams, this role will involve the writing of validation documentation as well as testing and analysis. Some companies call this role.
- Process Validation Engineer – This is generally considered an advanced role and requires thorough process knowledge and experience with process validation engineers working in engineering design, tech transfer, upstream and downstream manufacturing. Most practitioners tend to have a lab/science, chemistry or chemical/process engineering background although or have learnt experientially on the job through extensive work experience.
- Cleaning Validation Engineer – Design and develop cleaning procedures for new products and manufacturing equipment and also investigate and conduct troubleshooting/root cause analysis of cleaning-related incidents, deviations for non-validated or underdeveloped cleaning procedures. This is a highly specialised role requiring in-depth knowledge of chemical cleaning processes so most practitioners tend to have a lab/science, chemistry or chemical/process engineering background or have learned experientially on the job through extensive work experience or on-the-job training. Some companies might call this role:
- Cleaning Validation Specialist
- QA Validation Specialist – Cleaning Validation
- QA Validation – Clean Hold Executor
- Computer System Validation Engineer – CSV is a process used to prove (and document) that a GxP computer-based system will produce information or data the way it is designed to and not perform in ways that weren’t intended. The CSV process is necessary when replacing paper records with electronic systems within highly regulated environments that directly impact public health and safety, such as pharmaceutical and medical device manufacturing. Its use makes sure that the system is completely accurate, transparent, reliable, robust, and tamper-proof. One of the biggest misconceptions of working in Computer System Validation – is that you need to be able to code or have a software background or be able to program a PLC. This is not the case as CSV is about managing data accuracy, reliability and integrity, not programming. Some companies might call this role:
- Cleaning Validation Specialist
- CSV Specialist
- CSV Support Analyst
- CSV Subject Matter Expert
- CSV Lead
- Validation Team Leader – working to complete the validation plan, with supervisory responsibilities over other team members
- Validation Manager/Lead – head of a validation team with overall responsibility for the successful completion of validation activities
Your pathway to joining & progressing within the validation team
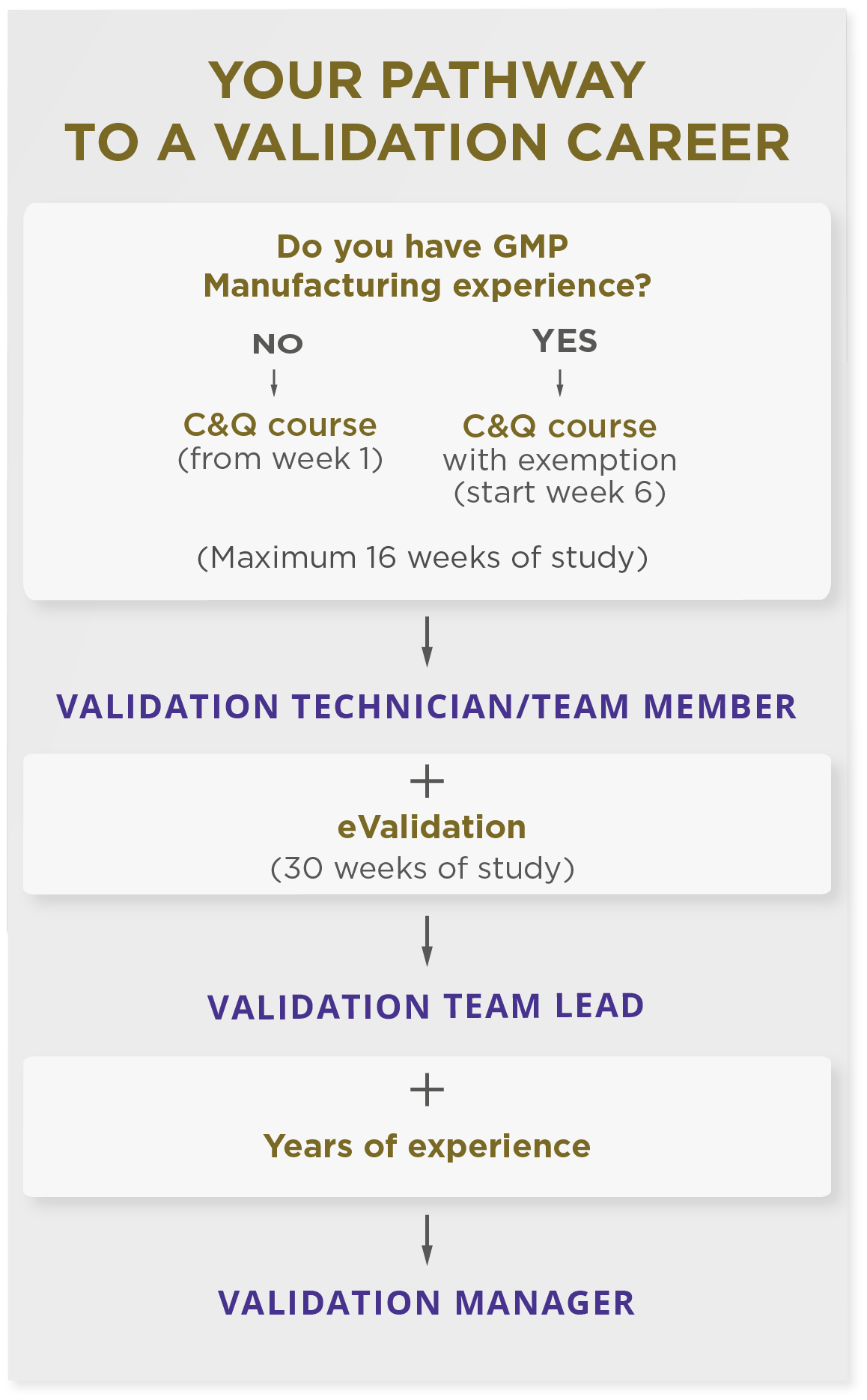
How to start your validation career
Most people start their validation career at the technician/team member level. But you can’t just join and learn on the job, you will need a course to explain the basic principles of validation and how to complete the documentation.
With no validation experience…
If you don’t have any previous experience in validation, check out our Equipment Validation Training Course for starter validation, CQV and C&Q roles.
By the end of this 15-week program you’ll:
- Be familiar with the rules governing GMP regulated environments
- Have developed your own 100-page plus equipment validation protocol
- Be able to read the engineering documentation necessary
- Be confident in how to qualify equipment, instruments and piping systems.
Here are the most common entry-level positions we see advertised.
- Validation Technician – Some pharma companies call this role:
- Associate QA Validation Specialist
- Equipment Validation Specialist
- QA Validation Associate
- QA Validation Specialist
- CQV Engineer – Some pharma companies call this role:
- CQV Specialist
- C&Q Specialist
- C&Q Junior Project Manager
With validation, CQV and C&Q roles, you could work directly for:
- pharmaceutical companies on in-house projects
- engineering consultancies as part of a project team on small to large capital projects
- engineering contractors as part of a project team on small to large capital projects
If you have experience in a GMP-regulated manufacturing environment, you may qualify for an exemption for the first 5 weeks of the program (and the associated discount). You’ll join the course in week 6 and complete the remaining 10 weeks of the program.
With validation experience…
If you already have some validation experience and can comfortably populate an equipment validation protocol, check out university-accredited Pharmaceutical Validation Training Courses for more senior validation, CQV and C&Q roles and move into roles that are more challenging and pay more. This is an intermediate to advance course where you will develop process validation protocols, plan a validation strategy and become a validation professional.
This program is university accredited at level 7 by Technological University Dublin, Ireland.
Over 30 weeks you’ll:
- Become familiar with pharmaceutical facility design
- Learn how to develop a validation masterplan
- Build a portfolio of validation projects and assignments that you can take to interview.
Moving into management…
A move into the most senior roles within a validation team is usually only possible after several years of experience within validation roles.
Don’t worry if you’re not sure. Whichever course you apply to, we’ll take a detailed look at your experience and give you a recommendation. *
* Please note that this recommendation might not be the course you applied to. If that is the case, it’s always because the recommended course is the right one for you. Our primary goal is always to get students to an “employable” level of knowledge in the shortest time.
Validation Salaries
Validation roles are well-paid. Even when compared against other salaries in pharmaceutical manufacturing (which themselves, are higher than comparable jobs in other industries).
Check out our validation salary guide for more details about validation salaries in your area.
About the Author
Claire Wilson
Content Marketing and Career Coaching
Claire runs GetReskilled’s Advanced Career Coaching Programme – our specially devised job hunting course that helps our trainees take that final step into employment by leading them through the job hunting process. She is extremely enthusiastic about helping people reach their final goal of employment in their new career path.
Claire has a BSc (Hons) in Medical Biology from Edinburgh University and spent 7 years working in the pharmaceutical and medical device industries.
