

Finding the right course when you’re changing career is a big decision. Your first thought will always be to find one that gives you enough information to get your foot in the door of the industry. Enough to convince an employer to give you a job.
But what about the longer-term? Wouldn’t it be great if that course could also be the first piece of an even bigger puzzle that could ultimately see you in a highly specialized and sought after role IF that was something you later decided you wanted to pursue?
Whatever your intentions, we’ve got the course path for you. But it can be a little tricky to see the big picture when every course outline and syllabus is packed full of words you don’t even know yet.
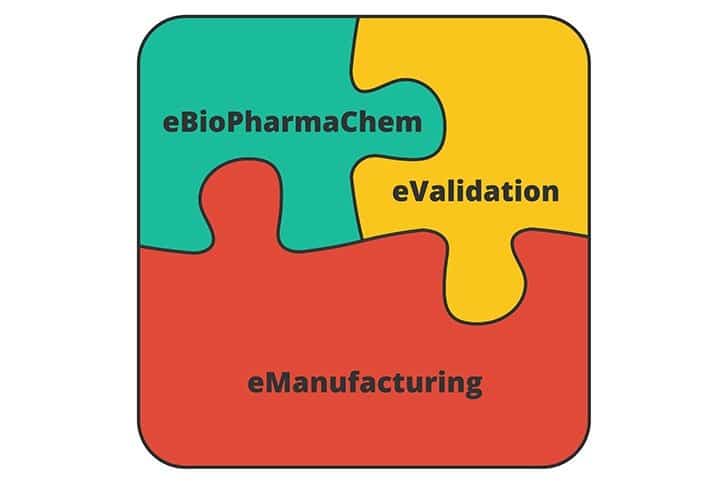
So let’s try this instead. This jigsaw puzzle represents our BSc degree – when you have all the pieces, you have a university accredited BSc qualification.
Certificate in eBioPharmaChem
Your path to a mid-career change into pharmaceutical or medical device manufacturing begins with our Certificate in eBioPharmaChem.
(That is, unless you have no industry experience or a scientific or engineering background. If that’s the case we recommend our 16-week Conversion Course into Pharmaceutical Manufacturing to get you on a level playing field at the start of eBioPharmaChem.
The Certificate in eBioPharmaChem is a Level 7 CPD Certificate that is university accredited by Technological University Dublin Ireland. It is an online course of 3 modules, taking 14 hours per week over 37 weeks in total.
Your Level 7 Certificate is worth 15 ECTS credits.
At the end of that, you will have the knowledge you need for an entry-level position in pharmaceutical or medical device manufacturing. In fact, many of our trainees manage to secure their first job in the industry while still studying this course. They go on to finish the certificate as they work.
You can quite happily leave your education after you get this certification.
Certificate in eValidation
If you decide, however, that you’d like to aim for jobs in the very sought after niche of validation, you can continue your education with a second programme, Certificate in eValidation.
The Certificate in eValidation is a Level 7 CPD Certificate that is university accredited by Technological University Dublin, Ireland. It is an online course of 3 modules, taking 14 hours per week over 30 weeks in total.
Your Level 7 Certificate is worth 15 ECTS credits.
This course equips you with the knowledge you would need to become a validation professional. (Sorry, we’re back to using industry-specific words again, have a look at this post on “What does a Validation Engineer do?“ to see exactly what validation is all about).
Jobs in validation are more advanced than the entry-level ones available to you after the Certificate in eBioPharmaChem. They generally pay more and, currently, there is a shortage of validation professionals in the industry so it could be a great time to get yourself these skills. Being a part-time course, you can study while you work.
Again, after earning this certificate, you could quite happily leave education behind and enjoy your new job in pharmaceutical or medical device manufacturing. If you’ve been with us from the start then you’ve got TWO Level 7 CPD Certificates that are university accredited and you’ve given yourself a great start in your new industry.
BUT…
You’re actually now halfway to a BSc degree.
BSc Manufacture of Medicinal Products
Our Certificate in eBioPharmaChem and Certificate in eValidation are standalone programmes. You can study one or both and earn accredited certificates.
But when the ECTS credits of these two courses are considered in combination – you have earned half of the credits needed to gain our Bachelors of Science degree (BSc).
If you’ve taken both programmes, you can then go on to study our eManufacturing modules.
eManufacturing is represented by the red piece of our jigsaw puzzle up above. You can how the 6 modules of that programme complements the learning of our Certificate in eBioPharmaChem and Certificate in eValidation to make up the BSc degree programme.
The eManufacturing modules are also delivered via an online part-time course that you can take while you work. Over 2 years, you’ll learn even more about the processes involved in manufacturing safe medicines and medical devices.
Upon completion of the eManufacturing modules (and assuming prior completion of eBioPharmaChem and eValidation), you will earn a BSc Manufacture of Medicinal Products, university accredited by Technological University Dublin, Ireland.
Inbuilt Flexibility
The best thing about university accredited programmes complimenting each other in this way is that you never have to over-commit. You’re only ever signed up for the course you’re doing at that time. If you want to move on to work toward the next piece of the puzzle, you enroll once you finish the previous one.
This means you can take a break in between courses or you can move straight from one to the next – whichever you choose. There is a time limit for overall completion of the 12 modules though, ask your course advisor if you have any queries about this.
Our courses are industry recognized qualifications in their own right. But they are also pieces of a larger puzzle – you choose how far you want to build your education.
So whether you just want a “foot in the door” after a mid-career change or you’re looking to take your new career to another level, and down a path of specialization, it all starts with the same first piece. No time you spend studying is wasted.
Other Useful Articles
You might also be interested in:
Ireland’s No.1 Pharma Job Search Resource Centre
Find over 40 resources, tools and templates to help you find a job in the pharmaceutical and medical device manufacturing sector in Ireland.
UK’s No.1 Pharma Job Search Resource Centre
Find over 40 resources, tools and templates to help you find a job in the pharmaceutical and medical device manufacturing sector in the UK.
Post Your Comments Below