Good Manufacturing Practices (GMP) are a set of product quality regulations ensuring that manufacturers and packagers of medicines and medical devices produce safe, pure, and effective products.
GMPs cover all aspects of production, including raw materials, factory and equipment maintenance, manufacturing processes, personal hygiene, training, packaging, labelling, record keeping, personnel management, product testing, and handling errors and complaints.
Failure to comply with GMPs can lead to severe consequences, such as product recalls, seizures, fines, and even jail time.
In this article, we will explore GMPs in detail and address common misconceptions and misunderstandings about their use and application on the factory floor.
BTW, if you want to get a solid understanding of GMP rules, regulations and guidelines, check out our 6-week GMP Training For Beginners in the Pharmaceutical Industry.
Table of Contents
- What are some things a GMP Manufacturing must do?
- Why are GMPs Important?
- What are the Different Types of GMPs?
- The Regulatory Authorities
- Cross Recognition of Regulation
- Licences and Authorisations
- People can’t be GMP certified. Nor can companies
- Regulatory Inspections
- What is the difference between GMP and cGMP?
- What are GxPs?
- What is a Quality Management System and how does it fit together with GMPs?
- How does ISO 9001 compare with GMPs?
- Do all the ingredients and materials that go into a finished drug product need to be manufactured under the control of GMPs?
- How do I get started using GMPs within a manufacturing plant?
- 18 rules of working on the factory floor in a GMP Manufacturing plant
- The 12 Principles of GMP
- Rule #1 Get the factory design right from the start
- Rule #2 Validate the design and processes
- Rule #3 Write good standard operating procedures (SOPs)
- Rule #4 Identify who does what
- Rule #5 Ensure SOPs and work instructions are being followed
- Rule #6 Keep good documentation and records
- Rule #7 Maintain facilities and equipment and storage
- Rule #8 Prevent contamination through cleanliness
- Rule #9 Train and develop staff
- Rule #10 Build quality into the entire production process
- Rule #11 Use quality risk management tools
- Rule #12 Perform regular quality audits
1) What Are Some Things a GMP Manufacture Must Do?
Good Manufacturing Practices (GMP) establish minimum standards for manufacturers of pharmaceuticals and medical devices. Each manufacturing plant must:
- Set up a robust quality management system
- Have detailed written procedures for processes affecting product quality
- Use high-quality raw materials
- Establish procedures for consistently manufacturing safe products
- Detect and investigate quality deviations
- Maintain reliable testing laboratories
- Document that employees follow correct procedures at every manufacturing step
GMPs ensure that patients receive the right product at the right strength, free from contamination, correctly labeled, sealed, protected against damage or tampering, and stored properly.
GMP regulations minimize contamination, mix-ups, and errors, protecting patients from ineffective or dangerous products. They emphasize that quality must be built into every step of the process, from development to manufacturing, distribution, and storage, rather than relying solely on testing.
GMP requirements are generally broad, allowing manufacturers to implement necessary controls and interpret regulations in a way that makes sense for their specific processes while focusing on patient safety.
2) Why are GMPs Important?
Medicines change the way the body functions, so the public expects them to be safe, effective, and consistently manufactured to high-quality standards.
With most other products, users can often spot problems before use. This is not true for medicines. It’s almost impossible for a medical professional or patient to determine if a medicine is safe just by looking at, touching, or smelling it.
The risk is greater because taking or injecting medicines directly into the bloodstream bypasses the body’s natural defenses, leaving us vulnerable to any manufacturing errors or impurities.
Therefore, the public, patients, and medical professionals need confidence that:
- The manufacturer produces medicines with high precision and safety.
- Every step has been taken to ensure quality, without shortcuts in the manufacturing process or ingredient sourcing that could affect the strength, quality, or purity of the final product.
3) What are the Different Types of GMPs?
The United States, European Union, Japan, China, India, Korea, the UK, World Health Organisation (WHO), etc all have their versions of GMPs.There are also standards like the International Conference on Harmonisation (ICH) and the Pharmaceutical Inspection Co-operation Scheme (PIC/S) that aim to harmonize GMP requirements across countries and promote cross-recognition of standards.
Many countries adopt one of these established sets of regulations to avoid the expense of developing their own. The specific regulation a manufacturer must follow depends on the location of the manufacturing plant and also, the market where the medicine or medical device will be sold. The ultimate goal of GMPs is always to prevent harm to the end user.
4) The Regulatory Authorities
In addition, each country has an organisation or agency that is responsible for the safety and efficacy (i.e it works) of the drugs that are manufactured and sold within its jurisdiction. The regulatory authorities will grant a license to a manufacturing plant to make a product based on that facility’s adherence to the relevant GMP regulations and enforce GMP regulations.
The regulatory authority may create its own GMP regulations and enforce them on any manufacturing plant supplying medicines to the public within its jurisdiction. For example, in the United States, the FDA (Food and Drug Administration) enforces its own 21 CFR Part 211 GMP regulations. The European Union has the EMA (European Medicines Association) which works together with the local regulatory authorities in each of its 27 member states who regulate their local manufacturers using the EU’s Eudralex regulations).
Even though the United Kingdom has left the EU, GMP regulations are still harmonized across Europe and UK manufacturers of medicines continue to work to the EU’s Eudralex regulations.
In addition, it’s common for a regulatory agency to audit and inspect a plant located outside its jurisdiction if that plant’s products are going to be supplied to the public within its jurisdiction.
For example, there are a number of plants in Ireland, India and China which are inspected and audited by FDA inspectors as the products manufactured in those plants are going to be sold within the USA.
5) Cross Recognition of Regulation
In the not-too-distant past, a single manufacturing plant supplying products to markets in Japan, Korea, the EU, the USA, Canada, etc., would often be audited by regulatory authorities from each of those countries.
The plant would find itself in a state of being constantly audited. Needless to say, this was incredibly expensive and wasteful. To reduce regulatory costs and burdens, agencies have been developing mutual cross-recognition agreements.
For example, if a plant in Japan is authorized by the PMDA (Pharmaceuticals and Medical Devices Agency), other regulatory agencies will recognize that authorization, eliminating the need for them to audit the factory separately.
6) Licences and Authorisations
Once a new medicine has successfully completed its clinical trials, the relevant regulatory authority will grant it a “Marketing Authorisation or Product Licence”. This means that the medicine is approved for use.
In addition, the manufacturing plant where the medication is going to be made must have and maintain a “Manufacturing Authorisation” or “Manufacturing Licence”. To obtain this authorisation or licence or regulatory authorities must audit and approve the pharmaceutical plant where the medication is made.
7) People can’t be GMP certified. Nor can companies
An often misunderstood point is that pharmaceutical or medical device companies are not approved to make a specific product at all of their manufacturing plants. Instead, regulatory authorities approve a specific product to be manufactured at a specific plant.
This means local regulators (MHRA, FDA, etc.) have visited, inspected and certified that facility to manufacture the product,it to manufacture that product conditional on demonstrating strong regulatory commitment and compliance to the relevant GMP standards.
GMPs focus on the outcomes of the facility as a whole, not on the actions of individual employees. Thus, neither the people nor the company are certified—only the manufacturing facility is certified to produce a specific product.
Sometimes, especially in North America, people say a product is “GMP Certified.” This means the product was made in a GMP-approved facility.
8) Regulatory Inspections
Regulatory authorities conduct routine and surprise inspections of sites that manufacture medicines to ensure they comply with Good Manufacturing Practices (GMP). They check if the plants have the necessary facilities, equipment, and capability to manufacture the intended drugs.
If a company fails to comply with GMP regulations, it can face severe consequences, including restriction, suspension, or forfeiture of manufacturing rights, product recalls, seizure of goods, fines, and even jail time.
Inspections typically occur every two to three years. If inspections reveal issues, the site could receive a warning or be closed. More frequent inspections may occur if authorities have concerns.
9) What is the difference between GMP and CGMP?
- GMP (Good Manufacturing Practices): This is the global term used by most regulatory authorities around the world (e.g., the European Medicines Agency, PICS, the World Health Organization, etc).
- CGMP (Current Good Manufacturing Practices): This is the term specifically used by the FDA in the United States. The “current” in CGMP does not mean that these practices are more up-to-date than international GMP standards. It’s just part of the terminology the FDA uses to emphasize that manufacturers must always adhere to the most current standards as they evolve over time.
Most countries don’t use the ‘c’ for ‘current’ as it’s assumed that manufacturers will keep up to date with changes to the GMP guidelines and will strive to meet them. And so for most practical purposes the terms cGMP and GMP are largely interchangeable terms.
10) What are GxPs?
GxPs is an umbrella term for several “Good Practice” frameworks which includes “GMPs”. These frameworks were created to make sure that high levels of quality and safety are built into every step from drug development and manufacture to distribution and storage as we don’t just want only the manufacturing process to be safe. We need these other steps and processes to be safe as well.
These include:
- Per Clinical Development: Good Laboratory Practice (GLP)
- Drug trials: Good Clinical Practice (GCP)
- Manufacturer: Good Manufacturing Practice (GMP)
- Distribution: Good Distribution Practice (GDP)
- Storage: Good Storage Practice (GSP)
And collectively, all these standards are known as GxPs.
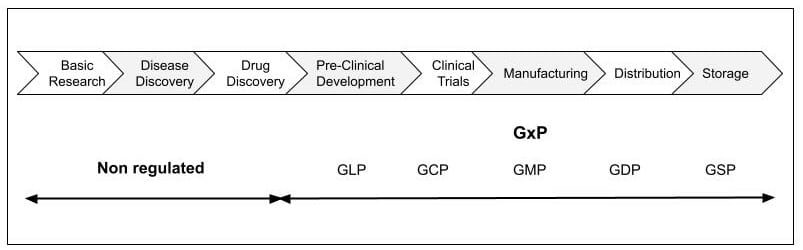
Lifecycle overview showing where GxP and GMP is implemented.
11) What is a Quality Management System and how does it fit together with GMPs?
A quality management system documents a company’s processes, procedures, and responsibilities to help it provide a quality product or service. It helps coordinate and direct an organisation’s activities to meet customer and regulatory requirements.
Arguably the most recognised and widely implemented quality management system is the ISO 9001 standard. This standard is published by the ISO organisation based in Switzerland and provides a set of requirements for an organisation regardless of what it does or its size. It’s a tried and tested framework for managing the organisation’s processes so that they consistently turn out products that satisfy customers’ expectations.
A company that uses ISO 9001 can have itself audited by the ISO organisation and publicly state that it is “ISO 9001 Certified” or “ISO 9001 Registered”. However, certification to the ISO 9001 standard does not guarantee compliance with GMPs (and therefore the quality) of end products and services. Rather, it certifies that consistent business processes are being applied. That is all.
12) How does ISO 9001 compare with GMPs?
While GMP regulations and ISO standards are often compared, the two are not the same and are not really trying to do the same thing.
Think of GMPs as regulations and legal requirements that pharmaceutical manufacturers must comply with. Think of the implementation of a quality management system such as ISO 9001 as a framework you can use to achieve compliance with those regulations and legal requirements
GMP is a product quality regulation that has the force of law behind it. Its focus is on getting product efficacy, (i.e it works) and manufactured to a high-quality standard for the most important customer of GMPs – the patient. If your manufacturing facility doesn’t strictly follow GMPs, the local regulatory authority can compel the plant to make changes, recall products, or even halt production.
ISO 9001 is a tried and tested quality management system that is regularly used by pharmaceutical manufacturers to help meet its GMP regulatory requirements. ISO 9001 is also more about running the whole business, a goal of which will be producing products of the right quality – but it has other aims too. Whilst GMP focuses on regulating production and quality control – ISO 9001 focuses on all departments and processes of an organisation.
13) Do all the ingredients and materials that go into a finished drug product need to be manufactured under the control of GMPs?
Yes. However, the GMPs for excipient fillers in a tablet, are different to the GMPs for the APIs (Active Pharmaceutical Ingredients) in the same tablet, which are different to the GMPs for active ingredients in an injectable.
These in turn are different to Water for Injection (WFI) which is added to the same injectable, which are different to the GMPs for the blister pack materials or vials in which they are packaged for distribution to the patients, which are different to the GMP’s governing the product instructions for the patient who will be taking the medicine that are included in the packaging box.
14) How do I get started using GMPs within a manufacturing plant?
There is a vast difference in the level of understanding you need of GMPs depending on whether you work in the factory floor vs working in a quality assurance role or managment position. And so if you look at good manufacturing practices from various regulatory authorities from around the world, you could condense the principles into two perspectives.
Perspective 1
The Tactical level rules and guidelines for those working directly on the factory floor.
Lets take a look…
15) 18 rules of working on the factory floor in a GMP Manufacturing plant
Imagine you have to write a list of rules and guidelines for everybody who works within the manufacturing area in a GMP-regulated facility. What would they be?
Here is a list of rules and good practices when working on the factory floor:
- Make sure you’re using the correct procedure when performing any task.
- Always try to follow the standard operating procedure as tightly as possible.
- Accurately record information when you perform a task.
- Only perform tasks that you are trained and competent to do.
- Always report any mistakes and problems that occur.
- Work in a clean and tidy manner to reduce the chances of mistakes.
- Always check that you’re using the correct equipment and materials.
- Only go into areas you’re permitted to.
- Try to minimise your physical movement with the GMP-controlled area.
- Make sure you gown up properly wearing the correct clothing.
- Always use a pen when making an entry on a paper record.
- If you make a mistake, cross the error out with a single line so that the original can still be read and sign and date the error.
- Never eat or drink inside a GMP-controlled area.
- Never take shortcuts no matter how sensible they seem as it may lead to unintended consequences that you have not foreseen. Always follow instructions.
- Make sure equipment, materials and areas are clearly labelled and identified
- Work logically and in a manner that avoids mix-ups with the wrong materials
- Work to prevent contamination of one material and another
- If you’re ever not sure, ask for help.
Perspective 2
High-level principles for those working in the quality department and management that you can use to drive day-to-day operations.
Let’s take an in depth look…
16) The 12 Principles of GMP
If you were to look at the various GMP regulations from around the world, you could condense them into the following list of 12 items for the quality department and management. If fact, many of these principles would equally apply in any manufacturing industry, but they specifically apply to the biomedical manufacturing industries.
- Get the factory design right from the start
- Validate the system
- Write good standard operating procedures (SOPs)
- Identify who does what
- Enforce / Implement SOPs and work instructions
- Keep good documentation and records
- Maintaining facilities and equipment
- Prevent contamination through cleanliness
- Train and develop staff
- Build quality into the who product lifecycle
- Use quality risk management tools
- Perform regular audits
Rule #1 Get the factory design right from the start
Pharmaceutical and medical device manufacturers have to follow GMP regulations when manufacturing product. However, it’s going to be much easier to be GMP compliant if you get the design and construction of the facilities and equipment right from the start. For that reason, you should front-load your efforts on building quality processes into your design so that you can avoid production and quality problems from the start rather than trying to procedularise out the problems when the plant is up and running. As the saying goes, an ounce of prevention is worth a pound of cure as having to make changes to the plant once it’s up and running is going to be prohibitively expensive.
Gruman Aircraft Corporation (the maker of the Apollo lunar landing module) followed a simple rule of thumb when designing new aircraft in that, you can choose a new engine, a new airframe or a new avionics system but never more than one new system at the same time. With that in mind, here are a few simple design corollaries.
- Use of the shelf-proven parts.
- Minimise the part count.
- Leverage in-house expertise and outside supplier expertise. Try to maximise the use of seasoned engineering and science expertise as this can save a world of problems.
- Use risk assessment analysis of the design to try and visualise catastrophe before it happens.
Factory Layout
Lay out the production area to reduce the chances of cross-contamination and to avoid mix-ups and errors. For example, don’t have the final product passing through or near areas that contain intermediate products or raw materials.
- remove unnecessary traffic in the production area to minimise the chance of contamination
- segregate materials, products, and their components to minimise confusion and the potential for mix-ups and errors.
Design:
What you are making is going to determine a lot of the building and facility requirements. You may need to conduct a risk assessment analysis to identify the right materials and design features.
- Materials of Construction – Stainless steel piping, tanks, floor, wall, ceiling covering, etc
- Windows
- HVAC requirements
- Utilities
- Emergency power
- Access to site
Environment
You must control the air, water, lighting, ventilation, temperature, and humidity within a plant so that it does not impact product quality.
Make sure that:
- the facility has good lighting.
- temperature, humidity and ventilation are controlled
- interior surfaces (walls, floors and ceilings) are smooth, free from cracks and do not shed particulate matter
- interior surfaces are easy to clean
- pipework, light fittings, and ventilation points are easy to clean
- drains are sized adequately and have trapped gullies.
Equipment
The equipment should be:
- suitable for its intended use
- clearly labelled.
- easy to repair and maintain
- properly installed and calibrated at regular intervals
- designed and installed in an area where it can be easily cleaned
Rule #2 Validate the design and processes
Validation is a legal and regulatory requirement for the manufacture of medicines. You have to prove that equipment and processes consistently do what they are supposed to do before you start production.
So what is validation?
Pharmaceutical Validation creates a documented evidence trail (through rigorously checking and testing) to demonstrate that a system, procedure or process used in the production and testing of the pharmaceutical product:
- maintains compliance at all stages
- and leads to a consistent and reproducible result
There are many other definitions of validation but the essence of all these definitions seems to be “documented scientific proof of consistent performance“.
Pharmaceutical validation can be divided into two broad elements.
Equipment Qualification
Equipment Qualification (sometimes referred to as IQ OQ PQ) ensures that equipment operates as intended and is installed in accordance with the manufactures recommendation. New facilities and equipment, as well as significant changes to existing systems, all require equipment validation.
Equipment Qualification is split into several qualification stages. Here are the different qualification stages using a piece of equipment as an example.
- A User Requirement Specification (URS) states exactly what you want. For example, what you expect an item of equipment to actually do.
- Design Qualification (DQ) covers how the item will be designed, such as its materials of construction and operational capabilities.
- Installation Qualification (IQ) checks that you’ve got what you wanted and that the item has been installed correctly.
- Operational Qualification (OQ) checks that each individual function of the item performs as expected, such as, it runs at the required speed.
- Performance Qualification (PQ) checks and documents that the equipment and systems meet the users’ needs. It’s like Operational Qualification, as it tests the operational requirements of the equipment, but in this case, the equipment will be under load.
Process Validation
Process Validation involves gathering documented evidence to confirm a particular process performs consistently and meets predetermined specifications.
Cleaning Validation
Cleaning validation proves the equipment used to manufacture the medicine is clean and cannot contaminate the medicine that is made in it.
Computer System Validation (CSV)
This is a process used to test, validate and formally document that a regulated computer-based system does exactly what it is designed to do in a consistent and accurate manner that is secure, reliable and traceable.
Validation Master Plan
You need to ensure that all validation activities are well planned and clearly defined and documented in a Validation Master Plan, or VMP.
Change Control
You also need to put a change control system in place to document all changes to facilities, equipment, or processes that could affect product quality. You must assess all changes to see if they have an impact on product quality and the whole quality management system.
Rule #3 Write good standard operating procedures (SOPs)
Written procedures provide detailed step-by-step instructions for the operators and users. They promote consistency and reduce variability as they allow the same task to be performed in the same way by different people. They also act as a reference. If a change or an improvement is identified, having a procedure in place creates a clear starting point which can be improved upon or modified in a controlled manner.
Writing Good Procedures
Written procedures are only effective if they are followed correctly, consistently and at all times by everyone.
- Make a quick outline of the task before you start writing the procedure.
- Use clear, precise and simple language.
- Steps should be numbered clearly and individually to make them easy to follow. Remember that people don’t usually read procedures from start to finish; they tend to scan the document for keywords. You can reduce this tendency by numbering each step.
- Break the procedure into chunks. Use heading, tables, bullet points and diagrams where necessary as this makes the information easier to digest and follow.
- Use simple sentences and write in a conversational style.
Rule #4 Identify who does what
Individual responsibility should be clearly understood and defined in job descriptions and everyone should have an up-to-date training record. All employees should clearly understand what they have to do each day as it avoids misunderstandings and minimises the risk to product quality.
You should create a job description for each role to define:
- job title
- job objective
- duties and responsibilities
- skill requirements.
There should be no gaps or overlaps in responsibilities.
Create an organisational chart and display it on the intranet or a local notice board. This way everyone in the organisation can see who does what.
Rule #5 Ensure SOPs and work instructions are being followed
It’s all very well to have great written procedures in place but we also have to have documented evidence that we actually follow them consistently as it’s a GMP requirement.
It often happens that the steps described in a written procedure may not seem most efficient way of performing the task. Taking shortcuts may save time or make the task easier, but you should never deviate from a written procedure without the approval of a supervisor or the Quality Department.
There are two main reasons for this:
- Many shortcuts can lead to unintended consequences that you have not foreseen.
- Each step in a procedure has been included for a purpose.
The inclusion of a particular step may not be obvious to you but it may be there as a check for another stage of the process. Of course, we should always strive to improve the process and eliminate waste but don’t change procedures without assessing the impact on the entire process.
Rule #6 Keep good documentation and records
It is an essential part of GMP to keep accurate records. This not only covers the rules for paper-based documents and records but also computer-based systems too. Good records enable you to track all activities performed during manufacture from the receipt of raw materials, to the final product release and allows for an investigation in case anything goes wrong. Good documentation control helps convey that you are following procedures to any auditor and demonstrates that processes are known and under control.
Follow these guidelines to ensure that good record keeping is part of your everyday culture:
- Records must be completed at the time of action and paper-based records must be completed using a pen.
- Record all necessary information immediately upon completion of a task. Never trust your memory or write results on loose pieces of paper.
- Write your name legibly in ink. Remember that by signing records you are certifying that the record is correct and that you have performed the task as per the defined procedure.
- Corrections to entries in records should be crossed out with a single line (to allow you to read the original entry) and be signed and dated. Correction fluid cannot be used. Include a reason for the correction at the bottom of the page.
- Record details if you deviate from a procedure. Ask your supervisor or the Quality Department for advice should a deviation occur.
- Don’t document someone else’s work unless you are designated and trained to do so.
- Never assume that undocumented work has been properly completed – if it’s not written down then it didn’t happen!
Rule #7 Maintain facilities and equipment and storage
Regular maintenance prevents equipment breakdowns which could disrupt production and can be very costly so it’s crucial to set up and preventive maintenance plan to make sure the facilities and equipment remain in good working order and fit for use. And.
- All equipment should be routinely maintained and calibrated where necessary.
- You should have written procedures for both scheduled and emergency maintenance. These should outline who does the work, the tasks involved and any additional information required.
- It’s a GMP requirement to have a maintenance schedule in place with the frequency determined by the criticality of the equipment.
- Storage areas should be kept clean and dry and free from pests. You need to provide suitable and secure premises and equipment that keeps materials and product within the correct storage conditions so that it does not get damaged, deteriorates or is tampered with.
- Premises should be of adequate capacity and have suitable lighting so the task can be performed accurately and safely. The environment should also be monitored, especially the temperature. Deviations from set points must be acted on and documented.
- Materials and products should be stored in a manner that prevents mix-ups of different batches and materials.
Rule #8 Prevent contamination through cleanliness
Practice good hygiene
It’s critical to reduce the risk of product contamination to a minimum by putting a program in place to maintain the cleanliness of the cleanroom. Develop a program to meet the standards of cleanliness necessary for the product. As an example, you would have different cleaning standards for sterile products used in an operating theatre as opposed to products that you inject into your bloodstream.
Keep these practices in mind:
- Always practice good personal hygiene by washing your hands at regular intervals
- Wear the required PPE protective garments and follow the gowning procedures.
- Inform your manager if you are ill
- Minimise contact with product or product contact surfaces and manufacturing equipment
- Never eat, drink, smoke or chew in manufacturing areas.
- Always follow cleaning and sanitation procedures.
- Report any condition that may cause product contamination.
- Remove rubbish and waste materials, and store them appropriately.
Rule #9 Train and develop staff
GMP has a big focus on the training of all people who have an impact on product quality. It’s crucial that your staff know how to do their job right first time, every time.
You should provide training for all employees whose duties take them into production areas or laboratories, and whose activities could affect the quality of the product. The training should cover how staff are expected to deal with problems, deviations, investigations, and changes. You should also provide training in response to the installation of new equipment or negative trends and inspection findings, as well as any new regulatory requirements.
Job-specific training is normally done by departmental trainers but QA has a role in training as well. This usually involves making sure that all staff are trained in good manufacturing practice and the quality system itself.
Rule #10 Build quality into the entire production process
As previously mentioned, a key tenant of GMPs is that you can’t test your way into high-quality productions. Rather, you must build quality into the entire manufacturing system during all stages of the manufacturing process. But what does that mean and how does building quality into the entire manufacturing system reflect itself on the factory floor?
What that means is that you must establish effective controls across the entire production process to assure product quality.
Facilities
Facilities must be clean, tidy and dry. Be of sufficient space to avoid mix-ups, with all equipment and rooms identified as to what is currently occurring. Steps should always be taken to avoid cross-contamination of one material and another.
Warehouse and Storage
In the warehouse, materials must be checked on arrival to ensure that they are what was ordered in the first place, are in the right condition, are labelled correctly and have been transported correctly. Any problems must be reported and problem items segregated from good stock. Materials cannot be used until approved by Quality Control.
The temperature of the warehouse must be recorded and deviations from established limits reported. If items need to be held under refrigerated conditions, then they need to be moved to the correct storage location on arrival.
All materials must be labelled with their name, batch number, as well as the expiry date and status such as “on test” or “passed”.
Manufacture
At all stages during manufacture, the equipment and rooms used should be identified with the product name and batch number. Steps must be taken to avoid cross-contamination of one item and another. So, use the required ventilation equipment, keep doors closed, only open one container at a time, clean up any powder or spills and clean up when you’ve finished the job.
At all times, wear the appropriate protective clothing correctly. Follow the instructions exactly for any job recording what you do at the time. If any problems occur, if you make a mistake, if anything does not look right, or anything out of the ordinary occurs, then report this to your supervisor straight away.
Packaging and Labelling Controls
With packaging, the use of the wrong materials can result in the end-user being given an incorrectly labelled medicine and this can be fatal. Great care is therefore needed to ensure that you have the correct materials and the work area is completely free from the previous batch before the packaging operation starts.
A packaging line must be set up exactly as instructed. During packaging, in-process control checks check that the pack is satisfactory with materials separated and inspected if there is a problem. There needs to be good segregation between different activities to avoid mix-ups and at the end of the job, the line and area, are thoroughly cleared of finished material and unused stock.
Rule #11 Use quality risk management tools
In manufacturing, testing, and distributing pharmaceutical or medical device products as in life, there are a lot of risks. With QRM, we make extensive use of worst-case scenario planning to figure out what could go wrong and come up with steps to mitigate those risks.
Think of it as a way of visualizing catastrophe before it happens and coming up with preventive steps either by designing out the problem (preferred) or procedurilize-out the problem (last resort).
So it is essential to do risk assessments if you want to change something or when something has gone wrong. Things to consider when doing a risk assessment following a problem include:
- The risk to product quality, safety and efficacy
- The risk to the end-user
- The risk to the process, area or equipment
- The risk to other batches, as well as
- The risk to your own personnel who do the job such as health and safety risks
Rule #12 Perform regular quality audits
And finally, we need to make sure that we’re doing what we’re told to do in a quality management system and that we are following our procedures.
You have to ensure that our facilities are fit for purpose, that we are validating our work, that our people are trained, that the plant is clean and we are controlling the process parameters in order to yield a particular product of a certain quality.
You should also conduct in-house audits, or self-inspections, to ensure GMP compliance.
See the checklist below. It’s a good practice to undertake a self-audit a few times a year and to target different manufacturing areas and departments each time.
References
Essential Pharma Documents: GMP Question and Answers
http://pharmacydocs.blogspot.com/2016/05/gmp-question-and-answers-including-in.html
Essential Pharma Documents: May 2016
http://pharmacydocs.blogspot.com/2016/05/
The 10 golden rules of GMP
https://www.pharmout.net/downloads/white-paper-10-golden-rules.pdf
Gmp audit response template
https://regogugone.weebly.com/uploads/1/3/4/8/134891767/pevevujufugakelapimi.pdf
GxP Compliance checklist | Ideagen
https://www.ideagen.com/thought-leadership/blog/everything-you-need-to-know-a
Documentation and Records: Harmonized GMP Requirements – PMC
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3122044/
What is GMP | cGMP | Good Manufacturing Practice | ISPE | International Socie…
https://ispe.org/initiatives/regulatory-resources/gmp/what-is-gmp
What is the golden rule of GMP?
https://www.plianced.com/compliance-qna/question/what-is-the-golden-rule-of-gmp/
GxP Regulated Environments
https://www.elpro.com/en/learn/gxp-compliant-monitoring
ISO 9001 | UK | FQM Limited
https://www.fqmltd.com/iso-9001
Current Good Manufacturing Practice (CGMP) Regulations | FDA
https://www.fda.gov/drugs/pharmaceutical-quality-resources/current-good-manufacturing-practice-cgmp-regulations
About the Author
Donagh Fitzgerald
Head of Marketing & Product Development
Mechanical/Production Engineer
Donagh looks after the marketing and product development including the training and pedagogical elements of our programs and makes sure that all GetReskilled’s users can have a great online learning experience. Donagh has lived and worked in many countries including Ireland, America, the UK, Singapore, Hong Kong and Japan. Donagh has also served as the Program Manager for the Farmleigh Fellowship based out of Singapore.
Donagh holds Degrees in Production Engineering and Mechanical Engineering from South East Technological University, Ireland.
Gerry Creaner
President
Senior Lecturer with GetReskilled
Gerry Creaner has over 40-years of experience in the Life Sciences Manufacturing industry across a range of technical, managerial and business roles. He established a very successful engineering consultancy prior to founding GetReskilled, an online education and learning business, with offices in Singapore, Ireland and Boston (USA), focussed on the manufacture of safe and effective medicines for the public.
He is also a founding Director of two Singapore based philanthropic organizations, the Farmleigh Fellowship and the Singapore-Ireland Fund, both of which deepen the well established and historical Singapore – Ireland relationship and deliver long-term benefits to both countries.
Gerry has an undergraduate degree in Chemical Engineering (UCD, 1980) and an MSc (Management) from Trinity College Dublin (2003) and is currently doing research for his Ph.D.



Post Your Comments Below