The forgetting curve refers to the loss of knowledge or skill over time when it isn’t reinforced or applied.
This presents a significant challenge for workplace and compliance training.
In regulated environments like pharmaceutical or medical device manufacturing, it’s typically measured by testing retention at intervals or observing performance lapses after training. In practice, this means an operator who learned a procedure may only recall a portion of it weeks later if there’s no refresher or practice in between.
Even the most engaging session can have a short-lived impact unless it’s followed by reinforcement – through practice, feedback, retrieval exercises, or real-world application. From a neuroscience perspective, this makes sense: lasting memory requires not just exposure, but transfer and consolidation, a process that depends on repetition, attention, sleep, and often, emotional relevance.
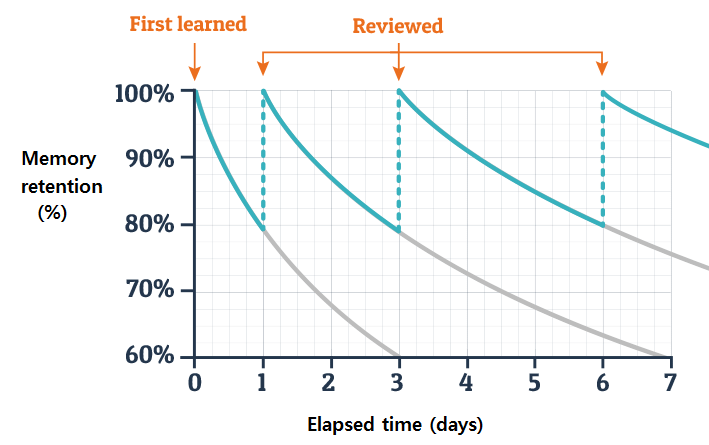
Researchers have long observed that memory tends to fade over time without reinforcement – a phenomenon first formally described by psychologist Hermann Ebbinghaus [1] in the late 1800s. His work led to the concept of the “forgetting curve,” which shows a steep decline in memory retention shortly after learning, particularly when the material isn’t actively reviewed or applied.
While exact figures vary greatly depending on the learner, the content, and the context, studies suggest that people can forget up to 50% of newly learned information within an hour, and as much as 70%–90% within a few days to a week if no effort is made to revisit or use the material.
Please treat these percentages with great caution though. These numbers aren’t universal – they come from controlled experiments with relatively simple or abstract information (like word lists or nonsense syllables) – but the underlying principle holds: our brains prioritise information that gets used, and discard what doesn’t seem necessary.
Cognitive Mechanisms Behind Decay
Why do people forget so much? One key reason is the way memory works: new knowledge is initially stored in short-term memory and must be transferred to long-term memory through processes like memory consolidation. Without reinforcement or practice, those neural connections weaken – a phenomenon Ebbinghaus quantified in his forgetting curve.
We also face interference: new information or daily tasks can override or confuse earlier learned knowledge. If training doesn’t engage multiple senses or contexts, it may form a shallow memory that’s easily lost. Conversely, techniques like spaced repetition and retrieval practice combat this decay [2] by strengthening memory traces over time. By intentionally recalling or applying information at spaced intervals, learners can retain much more over the long run (essentially “flattening” the forgetting curve).
Cognitive load is another factor – if too much is taught at once, employees may not encode it properly. Breaking training into smaller chunks and managing mental workload helps ensure critical concepts aren’t lost in a blur.
Educational research supports providing information in “small, manageable chunks with well-defined goals” to optimize retention. And remember, the size of the chunk of information depends on the individual’s prior knowledge of the subject and varies dramatically depending on your prior knowledge.
Contributors to Learning Decay in GMP Settings
In pharmaceutical Good Manufacturing Practice (GMP) environments, several factors accelerate learning decay:
- Cognitive: The sheer complexity of procedures (sterile technique, equipment setup, documentation protocols) can overwhelm memory. If employees only encounter a situation rarely (e.g. an infrequent sterility test or an annual calibration), the long gap causes natural skill fade. Lack of sleep or high stress (common in manufacturing shifts) further impairs memory retention and recall. Without frequent practice, even initially competent staff may “get rusty” on the finer points of SOPs. Studies in other industries show the length of the non-use interval strongly predicts skill decay; the longer you go without using a skill, the more it deteriorates
- Environmental: The workplace context can either reinforce or undermine training. Noise, distractions, and time pressure can cause people to skip steps they learned in training. If the training environment was very different (classroom lecture) from the real environment (fast-paced production floor), memory may not transfer well. Training classrooms might not prepare an operator for recalling instructions while sweating in a full-body gown in the cleanroom. Moreover, rotating shifts and high staff turnover mean knowledge isn’t consistently applied, leading to lapses.
Organizational: Perhaps the biggest culprit is a one-and-done training approach. If a company conducts training as a checkbox exercise (e.g. a long annual GMP presentation) without regular refreshers, it virtually guarantees decay. Organizational culture that doesn’t encourage questions or continuous learning will see employees’ knowledge stagnate. A “lack of ongoing training or low training frequency” is a common pitfall that regulators note in citations. If management assumes that once trained equals forever competent, they may miss the need to reteach employees. Additionally, poor training quality (e.g. dense slides, abstract information not tied to real tasks) leads to poor retention – learners can’t remember what wasn’t engaging or clear to begin with. As one industry expert put it, training that is just “Often e-learning is just an animated slide presentation that users march through without gaining real knowledge” will result in little to no lasting impact.
Timelines for Skill/Knowledge Degradation
The exact timeline for decay varies dramatically by role and task. Some knowledge can decay within days if not exercised – for instance, an employee might forget half of a GMP elearning content within a day or two without reinforcement. Most of the dramatic memory loss happens early (in the first hours and days), then tapers off. For manual or cognitive skills on the production floor, companies often assume that within a year of no practice, proficiency will significantly drop, which is why annual refreshers are a minimum standard.
However, critical aseptic techniques can degrade sooner; many firms require operators to retrain every 6 to 12 months in sterile gowning or aseptic manufacturing techniquess, recognizing that muscle memory and vigilance fade in that timeframe. In regulated pharma roles, an often-cited rule is “at least annual” GMP training, but this is more a compliance interval than a scientifically determined half-life of knowledge. In reality, if a manufacturing technician takes 6 months away (say on leave), their return-to-work retraining should be robust – significant decay can occur even over a few months of non-use.
On the other hand, tasks done daily (like routine Initial Production Checks (IPC) checks) will decay more slowly since if at all as they’re constantly reinforced on the job. The worst case is a rare emergency task that might be taught in initial training and not done for years (imagine an operator trained on an infrequently used piece of equipment or a rarely-invoked SOP).
In summary, many pharma companies pragmatically treat one year as the outer limit before some refresher training is needed, while acknowledging that for higher risk duties the interval should be shorter (e.g. quarterly or biannual brief refreshers) to prevent performance from sliding.
2: Impacts of Learning Decay in Pharmaceutical Manufacturing
In pharmaceutical manufacturing, training is not a one-time event. When knowledge fades over time, the consequences aren’t just deviations or regulatory citations. They can lead to compromised product quality and patient safety.
Employees forgetting key details – how to clean equipment, follow aseptic technique, or properly document batch records – can result in contamination, data integrity failures, or mislabeling. These aren’t theoretical risks; there are real-world examples where lapses in memory, not malice, led to recalled products or halted production. Even a forgotten glove sanitization step in sterile operations can seed microbial contamination in injectable drugs.
All too often, the root cause of a deviation is not “failure to follow procedure,” but failure to retain training. And while companies comply with training requirements on paper, that doesn’t guarantee retention or application.
Product quality depends on consistency. And consistency relies on human memory and behavior, especially in manual tasks.
Encouragingly, companies that implement frequent, focused refreshers – such as quarterly microlearning or targeted SOP reviews – can see measurable improvements: fewer human-error deviations, better data practices, and safer products.
3: Retraining, Upskilling and Organisational Strategies to Combatt the Forgetting Curve
Don’t Just Teach them About Something – Teach them to Do Something
One of the reasons training doesn’t stick is because it stops at theory. It’s way too abstract. The training (for example GMPs) focuses on facts and theory, not actions. People are taught what the rules are, what the regulators expect, what the acronym stands for but not how to apply any of it in real work.
This contributes directly to learning decay. If training isn’t grounded in real actions – what to say, write, record, or clean, people forget it faster. Information that doesn’t get used in context doesn’t get remembered.
To reduce decay, shift the focus:
- Don’t just explain the rule, have people apply it.
- Don’t just read the SOP, walk through the task.
- Don’t just define the term, simulate the situation where it matters.
People remember what they’ve had to do, not what they were told once in a slide deck.
Invest in Quality Training Design (“Telling Ain’t Training”)
Following on from the previous point, make sure that your training content and methods are effective. Simply telling or reading off SOPs is not truly training – if your internal program is mostly slide presentations or verbatim SOP reading, it needs an upgrade. Training needs to be practical and tied to actual work. The classic book “Telling Ain’t Training” emphasizes that people don’t learn well from just being told information in a lecture-style; they need engagement, relevance, and practice.
If you are an executive, you can support this by:
- Allocating budget for training development (e.g., creating simulations or purchasing a microlearning library) and by giving trainers the freedom to be creative.
- Attending or sampling training yourself, you can set an example that this is important and also see if they are truly engaging.
If currently your GMP refreshers are dreaded snooze fest, authorize a redesign – it will pay off in retention. Training should be linked with the organization’s goals. So insist that every training initiative has clear performance goals and an interactive design.
By improving training quality, you inherently reduce learning decay because people remember interesting, relevant training far better than boring, generic sessions.
Microlearning & Spaced Reinforcement
One of the most effective ways to prevent learning decay is microlearning [3] – delivering small, focused lessons (5 –10 minutes) at regular intervals. Instead of annual marathon sessions, microlearning spreads training throughout the year, aligning with how memory works: short, spaced reviews are far more effective at promoting longterm retention than cramming.
For example, a weekly 5-minute module covering a single GMP topic – like line clearance or data integrity – can be slotted into breaks or shift transitions. Some pharma firms pair these with quick quizzes or flashcards after formal training to reinforce key points. This approach builds a continuous learning culture and helps reduce human error caused by memory lapses.
Spaced repetition strengthens this effect by revisiting material at set intervals (e.g, 1 day, 1 week, 1 month later). Even simple reminders – an email summary, infographic, or short quiz – can act as “booster shots” that improve recall and performance.
Scenario-based training
Scenario-based training (SBT) is a learning method where employees are placed in realistic, job-related situations and asked to make decisions, solve problems, or take action – just like they would on the job.
Instead of passively watching slides or memorizing SOPs, learners are actively engaged in critical thinking and behavior-based learning. The goal is to simulate real challenges so employees practice applying knowledge, not just recalling it. This is especially important in pharmaceutical manufacturing, where regulatory compliance depends on demonstrable performance, not just training completion.
By seeing the consequences of their choices in a controlled setting, employees gain confidence, improve decision-making, and internalize the “why” behind GMP rules. This makes the training stickier, more relevant, and more aligned with day-to-day operations.
Example
Here is an example of a scenario we built to help validation engineers at their first day of work
You are a graduate validation engineer/technician on your very first day of work. You have to generate a validation protocol and deliver it on schedule on a new fast-track project. Take this decision-making simulation. Explore the various challenges you may encounter and see if you can avoid making 5 common mistakes on your first day.
Click on the bottom “Continue” tab to get started.
Immersive Based Training
Traditional retraining techniques (e.g., reading SOPs again) rarely improves performance. Immersive methods, like virtual reality (VR) can prove highly effective. These simulate real-world situations, allowing employees to practice and learn from mistakes in a risk-free environment.
A VR module might let a sterile operator practice gowning in a virtual cleanroom.
Cathy Moore’s Action Mapping and Relevance to Pharma Training
Cathy Moore’s Action Mapping is a well-known instructional design model focused on tying training to business goals and actions needed on the job. It’s easy to get into a mode of throwing information at trainees (the “tell them everything” approach).
Action Mapping flips this. It starts by asking:
- What are the measurable performance and business outcomes we want?
- What actions do people need to take to achieve those?
Then training is designed only around those necessary actions, with realistic practice for each. This prevents overload and irrelevant content that contributes to learning decay (because extraneous info is likely to be forgotten anyway).
For example, instead of a slide deck on microbiology, cleanroom training would focus on behaviors that prevent contamination like proper gowning or sanitization through scenario-based learning. The mantra is: “Design experiences, not information.”
This approach:
- Improves retention
- Aligns with regulatory expectations for demonstrable performance
- Avoids overwhelming learners with irrelevant details
By focusing only on what employees need to do their jobs well, this approach reduces cognitive load and makes training more engaging, efficient, and effective.
For training teams, applying this approach means always asking “How will this learning content help our staff do their work better or avoid errors?” If something doesn’t clearly tie to that, maybe it doesn’t need to be included.
Watch this video to get a sense of the approach where it walks you through a short discussion with a client, showing you how some quick questions can save you days of unnecessary training development.
Retraining Frequency by Role and Risk
Retraining should match the risk and complexity of the job – not just follow a calendar. While general GMP training is often annual, roles with high product impact (e.g., sterile production, critical cleaning) may require quarterly or semi-annual refreshers.
Beyond fixed schedules, use trigger-based retraining:
- After a deviation tied to knowledge gaps
- When SOPs or equipment change
- Following extended leave
- If audits reveal knowledge weaknesses
This ensures skills are refreshed when they’re most likely to have decayed or become obsolete. Regulatory guidance doesn’t mandate exact frequencies but expects firms to show that retraining keeps employees competent.
Beyond Multiple Choice: Assessing Real Competence
Multiple-choice quizzes are easy to administer and good for checking basic recall – but they fall short when it comes to assessing real-world GMP competence.
MCQs test recognition, not application. A technician might guess the right answer or memorize quiz content without truly understanding it. We’ve seen operators ace GMP tests, yet still make critical procedural errors. That’s because choosing the correct rule in a quiz isn’t the same as performing it under pressure.
Quizzes taken right after refresher training also fail to address learning decay. Someone might pass with flying colors today but forget key steps weeks later – creating a false sense of readiness.
Regulators expect more. FDA and EMA require that staff be qualified to perform their roles – which means demonstrating capability, not just passing a test. A quiz result may show training occurred, but if it doesn’t translate well to behavior on the floor, it’s of little value.
MCQs have their uses to be sure, but over-relying on them risks missing critical skill gaps. Real competency demands performance-based assessment – where employees show what they can actually do, not just what they remember.
Alternatives to assess real GMP competence include:
- Scenario-based decision making
Present a job-relevant situation – like a documentation error, equipment fault, or procedural deviation – and ask how the employee would respond. This evaluates their ability to apply GMP principles and make sound decisions in context. - SOP walkthroughs
Provide the employee with an SOP and ask them to walk through each step exactly as written, explaining what’s done, in what order, and why each step matters. This assesses their ability to interpret formal procedures and understand compliance-critical details. - Teach-backs
Without reference materials, ask the employee to explain a concept or task in their own words – as if teaching it to a new colleague. This reveals whether they understand the underlying principles and can communicate them clearly, beyond just following a checklist.
Group-based process simulations
Use diagrams, flowcharts, or mock batch records to simulate a multi-step process with a team. This tests systems thinking, collaboration, and the ability to spot issues across roles – especially useful for complex workflows or cross-functional processes.
Job aids
Job aids such as checklists, quick guides, decision trees, or embedded videos – are powerful tools that support employees at the moment of need. They are especially effective for infrequent or complex tasks, where retraining alone often fails to ensure retention.
Instead of relying on memory for rarely performed procedures, employees can follow a well-designed aid to complete the task accurately. For example, a step-by-step checklist can help an operator execute an annual cleaning validation without missing critical steps – something yearly retraining may not guarantee.
Some companies embed these tools directly into job workflows. In GMP settings, job aids may be integrated into electronic batch records via hyperlinks or short demo videos, shifting training from “just in case” to “just in time.” This approach reduces cognitive load, minimizes human error, and ensures consistency.
As performance expert Guy Wallace [4] notes, job aids “store information external to the user,” making them ideal for reducing reliance on memory – particularly for non-routine or high-risk tasks.
Watch this video from Atul Gawande from time stamp 12.51 on the importance and value of checklists.
Mentoring, Social Learning & Peer Support
People are key components of a learning ecosystem. Mentoring, peer learning, and coaching accelerate development and reinforce knowledge for both new and experienced staff.
Tactics include:
- “Buddy systems” for onboarding.
- Regular knowledge-sharing sessions or forums.
- Supervisor-led huddles with quick Q&As or mini-quizzes.
- QA and frontline managers addressing mistakes as real-time coaching opportunities.
These human interactions build a culture of openness—where employees can admit they forgot something and seek help, reducing the risk of error and enabling micro-relearning on the spot.
Blended & Reinforced Learning
Blended learning – combining instructor-led sessions, e-learning, hands-on practice, and reinforcement is more effective than one-off sessions alone. It boosts retention and better supports long-term performance.
The widely cited 70-20-10 model [5] suggests that:
- 70% of learning happens on the job
- 20% through coaching or mentoring
- 10% from formal instruction
While these figures aren’t based on hard science, the model serves as a useful metaphor for diversifying learning approaches. The 70-20-10 figure shouldn’t be taken literally or used to downplay the value of structured training especially in regulated environments. Still, the key message holds: great learning happens through a mix of experiences, support, and instruction.
To support continuous learning, reinforcement tools are essential.
- Microlearning platforms, LMS reminders, and gamified quizzes help keep knowledge fresh between formal sessions.
- Job aids—like checklists, quick-reference guides, or embedded videos—support “just-in-time” performance, reducing memory strain for infrequent or complex tasks.
For example, instead of retraining operators annually on a rarely performed task, a simple step-by-step job aid can guide correct execution in the moment. FDA and other regulators support the use of job aids as long as they’re controlled, current, and accurate because they improve compliance and reduce the risk of error.
4: Learning from High-Risk Industries:
The pharmaceutical and medical device manufacturing sectors can draw many lessons from high-risk industries that have long dealt with skill decay and human performance issues – such as aviation, nuclear power, and the military. These fields have developed rigorous approaches to ensure critical skills don’t erode.
Aviation
Airline pilots undergo simulator retraining and checks every 6 months [6] (or even more frequently for certain skills) to demonstrate they can handle emergencies and unusual situations. They practice things that might never happen in their normal flights, precisely so that if it ever does, they haven’t forgotten what to do. Pharma could adopt similar “drills” for crisis scenarios – e.g., a mock recall exercise or a simulation of a major equipment failure – to keep staff prepared for the unexpected. Aviation also uses extensive checklists (as discussed, akin to job aids) to back up memory, and promotes a culture of crew resource management where team members cross-check each other.
Nuclear industry
Nuclear plant operators are trained and re-tested frequently, and the industry has a strong blame-free reporting culture for near-misses to learn continuously. They emphasize procedure adherence strongly (like pharma) and also scenario simulation for disasters. Borrowing this, pharma companies might increase use of simulated scenarios as discussed (perhaps via VR or tabletop exercises) for things like contamination events or large-scale deviations, ensuring that if such an event occurs, the response is not being figured out from scratch by personnel who learned it years ago. Also, nuclear industry training programs often employ “overlearning” – training people past just the minimum proficiency until the responses are almost second nature – which helps combat decay because the deeper the initial learning, the longer it takes to forget.
Military
The military invests heavily in continual training, drills, war games, etc., recognizing that when not in active use, combat skills can fade. They also use adaptive training – for example, fighter pilots do dogfight simulations tailored to their skill level, increasing difficulty as they improve. The concept of train-as-you-fight (realistic conditions, stress inoculation) ensures that when the real situation arises, the training kicks in even if it was months ago. In pharma, while lives aren’t usually immediately at risk from a single error, patient safety is on the line cumulatively, so adopting a train-as-you-work mindset (realistic conditions, no classroom/real environment disconnect) would improve retention.
One specific high-risk technique used in some industries is error-based training: intentionally inducing or showing common errors in a safe training context and teaching how to recover or prevent them – this can reinforce memory by making the lesson very salient. For example, a training could demonstrate what happens if an aseptic operator breaches protocol to really drive home the importance; such visceral lessons often stick long term.
How to Get Started
If you’re looking for steps to implement:
- Shorten the gap between training and reinforcement.
Add a follow-up step—quiz, reminder, discussion—within a few days of initial training. This interrupts the forgetting curve and helps shift information from short-term to long-term memory. - Create job aids for tasks that aren’t done often.
Learning fades fastest when something isn’t used. Identify procedures performed infrequently but with high risk, and build checklists or quick-reference tools so people don’t have to rely on memory alone. - Switch one SOP refresher to a scenario format.
Repetition alone doesn’t prevent decay—application does. Instead of reviewing an SOP line-by-line, walk through a realistic situation where it would be used. This forces recall and builds retention. - Use trigger-based refreshers.
Set criteria for when retraining should occur: after extended leave, following an SOP change, or if a task hasn’t been performed in a defined time window. This targets moments when decay is likely, rather than retraining on a fixed schedule alone. - Build in spaced follow-ups for new content.
When rolling out a new process or change, plan short reminders over the following weeks. Even a 2-minute review at the next team huddle can dramatically improve retention if timed well.
These steps won’t eliminate learning decay but they can slow it, catch it earlier, and reduce the risk that forgetting turns into failure. Most importantly, they help turn training into something people remember and use – not just something they sat through.
References
[1] Ebbinghaus, H. (1885). Memory: A contribution to experimental psychology. Teachers College, Columbia University. (Translated in 1913 by Henry A. Ruger & Clara E. Bussenius)
[2] Cepeda, N. J., Pashler, H., Vul, E., Wixted, J. T., & Rohrer, D. (2006). Distributed practice in verbal recall tasks: A review and quantitative synthesis. Psychological Bulletin, 132(3), 354–380. https://doi.org/10.1037/0033-2909.132.3.354
[3] Thalheimer, W. (2017). Does microlearning work? What the research says. Work-Learning Research, Inc. https://www.worklearning.com/2017/10/10/does-microlearning-work-what-the-research-says/
[4] Wallace, G. (n.d.). Performance-based training & development. EPPIC Inc. http://www.eppic.biz
[5] Lombardo, M. M., & Eichinger, R. W. (1996). The career architect development planner (3rd ed.). Lominger Limited, Inc.
[6] Various. (n.d.). Training protocols in high-risk industries: Aviation, nuclear power, and military operations. Referenced from publicly documented industry best practices.
About the Author
Donagh Fitzgerald
Head of Marketing & Product Development
Mechanical/Production Engineer
Donagh looks after the marketing and product development including the training and pedagogical elements of our programs and makes sure that all GetReskilled’s users can have a great online learning experience. Donagh has lived and worked in many countries including Ireland, America, the UK, Singapore, Hong Kong and Japan. Donagh has also served as the Program Manager for the Farmleigh Fellowship based out of Singapore.
Donagh holds Degrees in Production Engineering and Mechanical Engineering from South East Technological University, Ireland.

Post Your Comments Below