Join over 2,000 people from all walks of life who have retrained with us and entered this industry
Past students include:
- Homemakers
- Self-Employed
- Early School Leavers – you don’t need a Leaving Cert or A Levels if you have work experience
- Working Parents – with young kids
- Restaurants/Hospitality – Chef, Cook, Restaurant Manager
- Healthcare – Nurse, Healthcare Assistant, Caregiver, etc.)
- Manufacturing – Production Operator, Assembler, Packaging, etc.
- Meat Processing – Operator, Butcher, etc.
- Defense Force – Army, Navy, Air Corps,
- Construction – Electrician, Carpenter/Joiner, Foreman, General Operative
- Warehouse – All Warehouse Workers
- Sales/Business – Retail Sales, Pharma Sales, Business, etc.
- Other – Farmers, Designers, Beauty/Hair Salon, etc
Pharma companies who hire non-technical people for operator roles can easily train them in weeks because:
- The core operator skills are procedural, not academic.
- Training continues on the job under supervision.
- GMPs requires specific site and product-level training. (It’s impossible to learn in a classroom)
Live Chat

Chat live with an expert
Hi, I’m Donagh Fitzgerald. I’ve spent over 14 years helping people figure out what kinds of jobs they can get in the pharmaceutical and medical device manufacturing industry based on their work experience.
Have a question? You can chat with me live any time – just click the blue chat bubble in the bottom-right corner of the page.
I’m online Monday to Friday during working hours (Irish/UK time).
If I’m away from my desk, just leave a message and I’ll get back to you as soon as I can.
What starter level jobs could I retrain for?
- Process Operator – Works in a pharmaceutical manufacturing plant such as Pfizer, AstraZeneca, Lilly, Abbvie, MSD, etc. Other names for this role are:
- BioProcess Operator
- BioProcess Manufacturing Operator
- Chemical Process Operator
- Cleanroom Operator
- Manufacturing Operator – Works in a medical device manufacturing plant such as Stryker, Boston Scientific, Medtronic, etc. Other names for this role are:
- Manufacturing Team Member
- Manufacturing Operative
- Product Assembler
- Production Operator
- Production Operative
- Production Team Member
- Packaging Operator – Works in both pharmaceutical and medical device plants. Some companies call this role:
- Packaging Operative
- Warehouse Operator
Estimated salaries from €32,000 – €42,000 with potential overtime, bonuses and shift allowances (Based on Irish job data).
For more senior jobs and a higher salary
Step 2: Take our award-winning Level 7 University Certificate in eBioPharmachem to give yourself a competitive edge, get more senior jobs with a higher salary and get a university qualification from Technological University Dublin Ireland.
Typical roles:
- Process Technician
- BioProcess Technician
- BioProcess Associate
- Manufacturing Technician
Estimated salaries from €45,0000 – €70,000 with potential overtime, bonuses and shift allowances (Based on Irish job data).
View Course Price
There are over 213 Pharma and Med Device Factories in Ireland employing 77,500 people

Are there Pharma and Med Device Factories and Jobs Near Me?
View UK’s largest pharmaceutical and Med device jobs board to see the latest job openings in the UK.
Find out what pharma and med device factories are near you – check out this factory locator for the UK to see what factories are within a commutable distance for you
Check out this list of 250 pharmaceutical and medical device companies
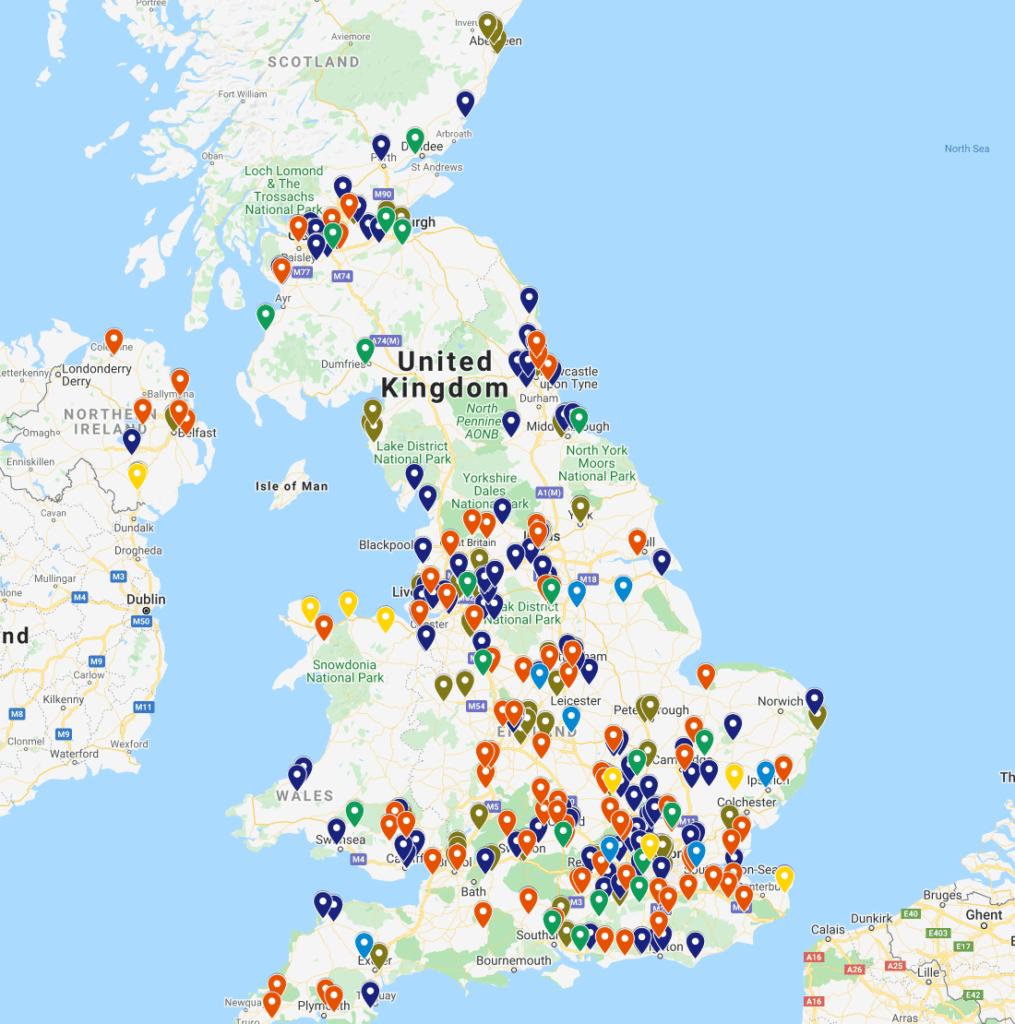
Are there Pharma Factories and Jobs Near Me?
Check out the Top 10 Pharmaceutical & Medical Device Industry Hubs in the USA
Common Questions About the BioPharma/MedTech Industry
Why Choose a Pharmaceutical Career?
Job Descriptions and Salaries
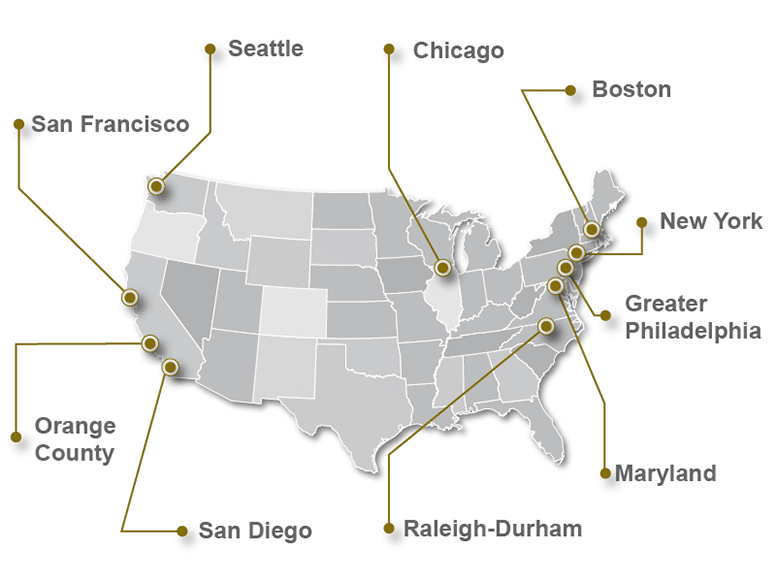
Common Questions About the BioPharma/MedTech Industry
Why Choose a Pharmaceutical Career?
Job Descriptions and Salaries
Your 10-Week Class Syllabus
This programme includes:
-
-
- ✅ Fixed weekly structure
- ✅ Weekly content release
- ✅ Progress monitoring
- ✅ Weekly deliverables
- ✅ End of module assignments
-
- ✅ Accountability coach
-
Module 1 – Manufacturing Safe Medicines and Medical Devices (GMP) (Weeks 1-6)
Module 2 – Advanced Job Hunting (Weeks 7-10)
Hear from people who’ve found a job in pharma after taking this program

Bharvi Soni
Previous Background:
Event Management
“I found the course interesting, challenging, rewarding and valuable”
“The course material was very relevant and the course coordinator was flexible and always approachable for any queries. I found the course interesting, challenging, rewarding, and valuable. After attending it, I managed to find a job in the pharmaceutical/medical device industry straight away. I am starting to apply the skills that I learnt from the course which will be a great contributor to my career. I already have, and will, recommend this course to others.”

Sean McLaughlin
Previous Background:
Operator (Pepsi)
“I have a new confidence going into the future”
“I really enjoyed the course, it gave me great confidence and taught me new skills, and most importantly, I am now working in a manufacturing facility. This is an excellent course not just for the insight into the biopharmaceutical industry, but it also gives you a great idea of who you are, and updating your CV and getting involved with social media, betters your chance of finding employment. This programme was hard at times but the help was there whenever it was required.”

Fergal O’Doherty
“This is an excellent course”
“The course gave me an understanding of key pharmaceutical concepts, document traceability and the science behind pharmaceutical products
This is an excellent course for those wishing to work in the pharmaceutical sector.
The online course was very enjoyable and has helped me on a day-to-day basis in my career”
Jessa Reid
Agnes Hove

Ross Miller.
Price
For 5 months
Or €2399 in advance
Price Includes:
- End of week progress checks by us to make sure you finish the program
- Delivered online so you can learn from home (or anywhere) on your own schedule
- Advanced job hunting program that will help you find your first job in pharma
- Regular & ongoing access to a dedicated career coaching counsellor
No Hidden Fees
- No application fees
- No registration fees
- No resource/book fees
- No certificate fees
For 4 months
Or $2699 in Advance
Price Includes:
- End of week progress checks by us to make sure you finish the program
- Delivered online so you can learn from home (or anywhere) on your own schedule
- Advanced job hunting program that will help you find your first job in pharma
- Regular & ongoing access to a dedicated career coaching counsellor
No Hidden Fees
- No application fees
- No registration fees
- No resource/book fees
- No certificate fees
Or £499/month for 4 months.
Price Includes:
- End of week progress checks by us to make sure you finish the program
- Delivered online so you can learn from home (or anywhere) on your own schedule
- Advanced job hunting program that will help you find your first job in pharma
- Regular & ongoing access to a dedicated career coaching counsellor
No Hidden Fees
- No application fees
- No registration fees
- No resource/book fees
- No certificate fees
Other Delivery Options
10-Day Fast Track Option with Priority Support (available for an additional fee)
- Complete this programme in 10 days.
- Your course leader will check your progress every morning and follow up with you to keep you on schedule.
- Upon receipt of payment, you can begin within 24 hours or choose a start date that works best for you.
- Contact us to get started.
Subscription Track (contact us for pricing)
- Best for: People with limited or changing availability who want significant flexibility in their course delivery.
- You can opt out of the subscription at any time, via email.
- Contact us for more details.
Price & Start Dates
Or $699/month for 4 months.
Price Includes:
- End of week progress checks by us to make sure you finish the program
- Delivered online so you can learn from home (or anywhere) on your own schedule
- Advanced job hunting program that will help you find your first job in pharma
- Regular & ongoing access to a dedicated career coaching counsellor
No Hidden Fees
- No application fees
- No registration fees
- No resource/book fees
- No certificate fees
Or €499/month for 5 months.
Price Includes:
- End of week progress checks by us to make sure you finish the program
- Delivered online so you can learn from home (or anywhere) on your own schedule
- Advanced job hunting program that will help you find your first job in pharma
- Regular & ongoing access to a dedicated career coaching counsellor
No Hidden Fees
- No application fees
- No registration fees
- No resource/book fees
- No certificate fees
Or £499/month for 4 months.
Price Includes:
- End of week progress checks by us to make sure you finish the program
- Delivered online so you can learn from home (or anywhere) on your own schedule
- Advanced job hunting program that will help you find your first job in pharma
- Regular & ongoing access to a dedicated career coaching counsellor
No Hidden Fees
- No application fees
- No registration fees
- No resource/book fees
- No certificate fees
Other Delivery Options
10-Day Fast Track Option with Priority Support (available for an additional fee)
- Complete this programme in 10 days.
- Your course leader will check your progress every morning and follow up with you to keep you on schedule.
- Upon receipt of payment, you can begin within 24 hours or choose a start date that works best for you.
- Contact us to get started.
Subscription Track (contact us for pricing)
- Best for: People with limited or changing availability who want significant flexibility in their course delivery.
- You can opt out of the subscription at any time, via email.
- Contact us for more details.
Price & Start Dates
Or £499/month for 4 months.
Price Includes:
- End of week progress checks by us to make sure you finish the program
- Delivered online so you can learn from home (or anywhere) on your own schedule
- Advanced job hunting program that will help you find your first job in pharma
- Regular & ongoing access to a dedicated career coaching counsellor
No Hidden Fees
- No application fees
- No registration fees
- No resource/book fees
- No certificate fees
Or €499/month for 5 months.
Price Includes:
- End of week progress checks by us to make sure you finish the program
- Delivered online so you can learn from home (or anywhere) on your own schedule
- Advanced job hunting program that will help you find your first job in pharma
- Regular & ongoing access to a dedicated career coaching counsellor
No Hidden Fees
- No application fees
- No registration fees
- No resource/book fees
- No certificate fees
Or $699/month for 4 months.
Price Includes:
- End of week progress checks by us to make sure you finish the program
- Delivered online so you can learn from home (or anywhere) on your own schedule
- Advanced job hunting program that will help you find your first job in pharma
- Regular & ongoing access to a dedicated career coaching counsellor
No Hidden Fees
- No application fees
- No registration fees
- No resource/book fees
- No certificate fees
Other Delivery Options
10-Day Fast Track Option with Priority Support (available for an additional fee)
- Complete this programme in 10 days.
- Your course leader will check your progress every morning and follow up with you to keep you on schedule.
- Upon receipt of payment, you can begin within 24 hours or choose a start date that works best for you.
- Contact us to get started.
Subscription Track (contact us for pricing)
- Best for: People with limited or changing availability who want significant flexibility in their course delivery.
- You can opt out of the subscription at any time, via email.
- Contact us for more details.
Application Deadline: Wednesday 19th November
Program Starts: November 26th
Application Process
The application process is an extremely easy 5 step process:
- Complete the application form - click on the big red "Apply NOW" button.
- Speak to a course advisor - they will let you know if you're a good fit for the program and answer any questions you have.
- Complete the registration form - you'll be sent this by email after you've spoken to a course advisor
- Make payment - successful applicants will then make payment. It is only at this stage that a place on the course is secured. Payments can be made in full or set up as monthly instalments to fit your budget.
- Begin your course!
We'll be right there with you every step of the way. If you have any questions, please get in touch!
Read our most recent independently verified reviews from “Google My Business” page
Read our most recent independently verified reviews from “Google My Business” page

Mihaela Olariu
Previous Background:
Operator (Cadbury)
"Highly recommended to anyone. This course was excellent"
“I found the course was run very professionally. The course notes and videos supplied were excellent, the notes tied in very efficiently and accurately with the videos.
Highly recommended to anyone. This course was excellent.”
Ines Doksa Grabrovec
Previous Background:
Supervisor (Fast Food Restaurant)
"It is hard work but it’s worth it"
“I’m a foreign person in this country with huge experience from my own country but somehow employers just did not recognise my qualifications. However, upon completing this course I finally feel more recognised.”

Edel Harkins
Previous Background:
Career Break (Full-time parenting)
"Highly recommended to anyone who has been out of the industry for a period of time"
“I would highly recommend anyone in my situation who has been out of the industry for a period of time to do these courses. They have contributed to my new found confidence in my existing and new qualifications, my improved interview skills and my new job!”
How We Deliver Our Online Courses without ZOOM Classes
With every GetReskilled ONLINE program;
- Centralized Platform: We use one platform (Moodle) where you can log into your classroom anytime. Each week, you’ll watch videos and complete quizzes, tests, interactive activities, and projects. The course materials are available 24/7, so you don’t have to be online at a specific time. There are No Zoom Classes. Study anywhere, anytime, such as after the kids are in bed or on the weekend.
- Flexible Schedule: Your working schedule may be unpredictable, so we offer flexible delivery. You can slow down, speed up, or pause the program as needed.
- Progress Checks: We release one week’s worth of material at a time and manually check your activity logs at the end of each week to ensure you are keeping up with your work.
- Dedicated Course Leader: Your course leader will:
- Help you create a weekly study plan
- Answer any questions
- Check your progress every Monday
- Follow up regularly to support you until the end of the course.
This all helps us to spot any potential issues early and helps you completely finish the program.
So let’s get started….
Get a better job with a higher salary.
Contact Details For This Course
Geraldine Creaner
Delivered by a practicing industry expert
Dr. Joe Brady
Full-Time Validation Lead
Lecturer, Technological University Dublin
Senior Associate, GetReskilled
Dr. Joe Brady is a full-time practicing Validation Lead and an assistant lecturer with Technological University Dublin (TU Dublin), in the School of Chemical and Pharmaceutical Sciences. Joe is a certified trainer and highly experienced in competency-based training. He designs and prepares educational modules and full academic programs ranging from MSc, MEngSc. BSc, to Certificate level, for a range of academic institutions.
He is also a supervisor for MSc/MEngSc and Ph.D. theses. Joe has over twenty years of project experience in the pharmaceutical, biopharmaceutical and medical device industries in Ireland, Singapore, China, The Netherlands, France and the USA.
Meet your online classroom support team
We have a team of in-house experts to provide guidance and support, whenever you need it.
Career Coaching – I will teach you to find a job

Meet Claire who runs GetReskilled’s Advanced Career Coaching Programme – our specially devised job hunting course that helps our trainees take that final step into employment by leading them through the job hunting process.
Course Leaders, Coordinators and Classroom Support

Your Course Leaders, Coordinators, and members of the Classroom Support Team are here to provide you with answers, tips, and are going to check your progress weekly to keep you on track and will reach out to you by email or even by phone if you fall behind!
Earn a Certificate of Award in the "Manufacturing Safe Medicines and Medical Devices (GMP)"
Earn the certificate by successfully completing two written end-of-module assignments and meeting all course deadlines in a timely manner.
Add details of your certificate to your CV/Resume or your LinkedIn profile.

So let’s get started….
Don’t settle with your current job and salary
Contact Details For This Course
Geraldine Creaner
Live Chat

Hi, I’m Donagh Fitzgerald. Chat with me live anytime – just click the blue chat bubble in the bottom-right corner of the page.
I’m online Monday to Friday during working hours (Irish/UK time).
If I’m away from my desk, leave a message and I’ll get back to you as soon as I can.
Or:
Send us a message
Fill out the form below and we’ll reply within one working day.
View Course Price
View Course Price
This 2- module program will teach you what you need to get your first job
Module 1 - Manufacturing Safe Medicines and Medical Devices (GMP) (Weeks 1 - 6)
After you complete this module:
- You'll be able to talk to employers with confidence about the systems and processes used in pharmaceutical and medical device manufacturing and about the rules and regulations that you need to follow.
- You’ll have enough technical knowledge to work in this environment.
-
You’ll be able to write reports and reference materials in a manner consistent with the professional norms of this industry.
Module 2 - Advanced Job Hunting For the Pharma Industry (Weeks 7-10)
After you complete this module:
- You’ll know about your local BioPharma/MedTech manufacturing industry and where the jobs are.
- You'll know which jobs you’d be perfect for and where to find them.
- You'll be able to assess your own skill set and know how to sell that to employers.
- You’ll know what employers expect from your application and job interview.
- You'll be able to follow a step-by-step process to find, apply and successfully interview for your first job in this industry.
View Full 10 Week Syllabus →
We do more than anyone to help people actually find jobs in pharma
Most universities and colleges hand you a certificate and wish you luck.
We didn’t think that was good enough. So we spent 10 years and thousands of hours building the most complete set of pharma job-hunting tools in Ireland.
More than every other university and college. Combined.
For example:
✅ A live, constantly updated pharma job board
✅ 40+ downloadable tools: pharma job CV templates, networking templates, interview prep
✅ A Google map of 213 pharmaceutical and med device factories in Ireland
✅ Table of 213 factories in Ireland segmented by county
✅ Guides to speculative job applications, factory outreach, and more
No other education provider comes close. If you’re serious about getting into this industry, we’ve built the clearest path for you to follow.




