What is IQ OQ PQ and why are they critical in the Pharmaceutical Industry?
By: Gerry Creaner B.Chem Eng. and Donagh Fitzgerald B.Prod Eng. Last Updated: February 2024
What is IQ OQ PQ in the Pharmaceutical Manufacturing Industry?
IQ OQ PQ stands for Installation Qualification, Operational Qualification, and Performance Qualification. These are 3 sequential processes used to confirm and document that the equipment, piping, instruments, and utilities (air, water, steam), etc are installed correctly, operate as intended, and perform consistently according to the design specifications.
The goal of these processes is to establish a documented evidence trail (paper or electronic) with multiple signatures from all relevant departments (Validation, Engineering, Maintenance, Calibration, Quality Assurance, etc,). This documentation proves to the (owners/clients or regulatory agencies) that the critical equipment ordered has been delivered, installed and configured correctly and that the system as a whole is working as per the engineering drawings and design specifications.
Here is a quick summary of each of the 3 processes…
Installation Qualification (IQ)
- This is the first step
- Checks and documents that the equipment, piping, inline components, or instruments you specified in the detailed design specifications have been:
- Properly delivered
- Correctly installed
- Configured according to standards set by the manufacturer or by an approved installation checklist
- Compares what was “specified” with what was “found” on site
Operational Qualification (OQ)
- OQ normally follows IQ
- Tests and documents that the equipment and systems operate as intended and are within the operating ranges listed by the manufacturer. For example, does it run at the required speed or heats up a liquid to the required temperature? Is the motor turning in the right direction?
- Confirms that the equipment runs the way it’s supposed to run.
Performance Qualification (PQ)
- PQ should normally follow the successful completion of IQ and OQ
- Confirms and documents that the equipment and systems are fit for intended use as defined in the user requirements specification (URS)
- Tests should cover the operating range of the intended process with the equipment under load
Each of these procedures can be standalone documents or combined (i.e. IQ and OQ can be combined into IOQ. In other situations, PQ may be combined with OQ or combined with Process Validation). However, in every case, the protocols need to be prepared specifically for the system or equipment being qualified.
If you need to learn how to develop an IQ IQ PQ equipment qualification protocol, check out our Equipment Validation (IQ OQ PQ) Training Course – For Starter Validation, CQV and C&Q Roles.
And if you would like a more in-depth look at IQ OQ PQ, keep reading…
Jump to section
- Why is IQ OQ PQ critical for product quality and patient safety?
- What is IQ or Installation Qualification?
- What is OQ or Operational Qualification?
- What is PQ or Performance Qualification?
- What is the V-Model?
- What is an Equipment Validation Protocol?
- How to write and develop the protocol
- How to execute the protocol
Why do we need Qualification (IQ OQ PQ ) in the pharmaceutical manufacturing industry?
Two main reasons.
1) The first reason is that it’s a legal requirement.
According to the Food and Drug Administration (FDA)
“Equipment used in the manufacture, processing, packing, or holding of a drug product shall be of appropriate design, adequate size, and suitably located to facilitate operations for its intended use and for its cleaning and maintenance.” – 21 CFR Title 211.63,
Do note that while the FDA deems equipment qualification essential in pharmaceutical manufacturing, it does not provide detailed instructions for how this should be carried out. Instead, it’s the responsibility of each company to ensure their equipment is well-designed, cleanable, and maintainable, and to prove its effectiveness and fitness for use. The exact methods used to qualify each piece of equipment, system or utility system are a choice made by each individual company.
Within the EU, EudraLex – Volume 4 – Good Manufacturing Practice (GMP) provides more detailed guidance on qualification under Annex 15: Qualification and Validation
And while not regulation, ISPE Baseline Guide 5 Commissioning and Qualification (Second Edition) is a widely used guide in qualification that enjoys the support of numerous regulatory authorities.
2) The second reason is that when qualifying and validating a new plant or process, even the slightest installation error or the most trivial problem with equipment performance can cascade and escalate into a serious product quality issue with deadly consequences for patients. This problem is especially acute with novel or new systems where there is zero track history of performance or failure and even tiny problems can lead to sick or dead patients.
So we use the IQ OQ PQ process to:
- Create a documented evidence trail to show that the mechanical, piping or software system is installed correctly, meets the design specifications and leads to a consistent and reproducible result under load.
- Help prevent installation, operational and performance errors from happening.
- Guarantee reliable performance.
Who does the qualification?
Qualification is usually done by the engineering group, the validation team or any other person or group that is qualified and knowledgeable on the use and operation of the equipment, and has the training and experience to perform the tasks required.
What is IQ or Installation Qualification?
Installation Qualification is a documented process that confirms that critical pieces of equipment, piping, software or instruments that directly impact product quality have been:
- Properly delivered (i.e you were shipped exactly what you ordered)
- Correctly installed
- Configured according to standards set by the manufacturer or by an approved installation checklist
Think of it as a process of checking and verifying a piece of installed equipment against a pre-prepared checklist to make absolutely sure it meets the design specifications and has been installed correctly. For example, if you have just installed a new pressure vessel, you want to make sure that:
- The manufacturer shipped you the right piece of equipment
- It’s made from the right materials as per the design specifications
- It is installed in the correct location
- The pipework, instrumentation or electrical wiring is connected up properly
This process is carried out by Commissioning, Qualification, Validation (CQV) engineers and technicians, Commissioning and Qualification (C&Q) engineers and technicians or Validation engineers or technicians.
IQ typically includes the following activities:
- Cross-checking of received contents against the packing list and checking for damage
- Verifying that the manufacturer’s technical specifications of the equipment matches the design and operational requirements
- Verifying that the serial number, make, model, etc. are identified for all components and are correct
- Checking the correct piece of equipment is installed in its proper location
- Verifying wiring or piping connections with other units or equipment
- Checking the installation of secondary instruments and ancillary equipment
- Checking for proper energy or utilities supply
- Confirming that the environmental and operating conditions are within the manufacturer’s guidelines
- Verifying software installation
- Recording firmware versions and serial numbers
- Recording calibration and validation dates of equipment used for the qualification
- Gathering all documentation, manuals, calibration certificates and certificates of conformity
This entire process is documented on pre-approved checksheets that are signed by the person performing the procedure and then approved and signed by a senior validation peer and quality assurance representative.
Layout of an IQ checksheet for equipment installation
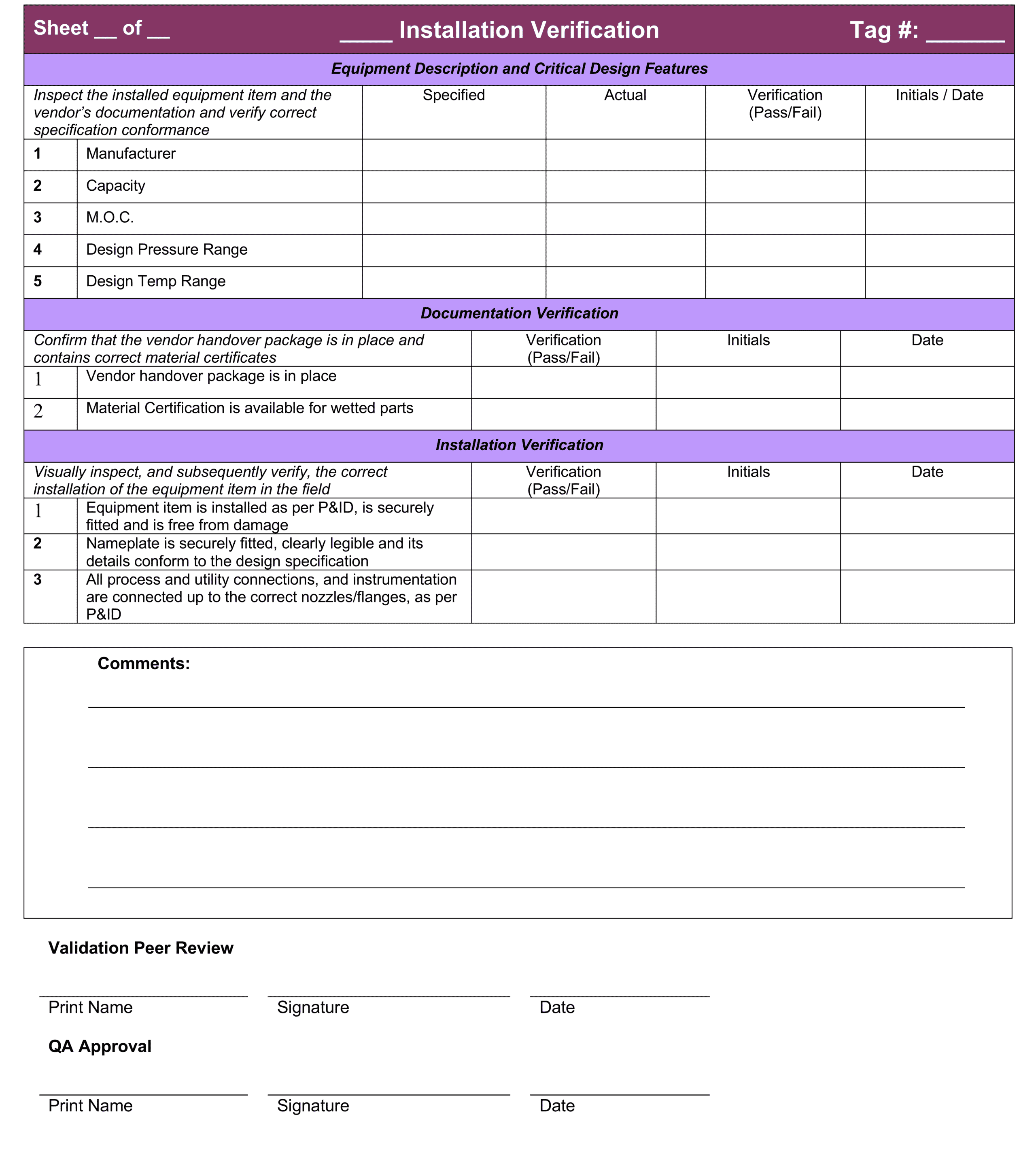
Click here for some more examples of professional IQ Templates for equipment installation verification, instrument installation verification, piping instrumentation verification and a P&ID walkdown template.
What is OQ or Operational Qualification?
Operational qualification is a documented testing process to make sure that the equipment and systems operate as defined in the design stage and are within the operating ranges listed by the manufacturer.
This is the next step and confirms that the equipment runs the way it’s supposed to run.
To carry this out, C&Q engineers must work with maintenance personnel, process, chemical, automation, instrumentation & mechanical engineers to follow a test plan. They test the equipment, document the performance, then compare and verify against the design specifications.
Features of the equipment that might be tested at this phase of the qualification include:
- Temperature Control Systems
- Overheating/Low-Temp Alarms
- Pressure Control Systems
- Fan/Motor Speed/RPM
- Display Units/Human-Machine Interface Units (HMI’s)
- Operational Signals
- Servo Motors Accuracy
- Pressure Switches
- Level Switches
Testing of these features may involve simple tests such as sending power to the unit and opening/closing different ports and valves to ensure signals or switches are functioning and that the components are in working order.
More complicated testing may need to be carried out, such as:
- Pressure Tests
- Flow Tests
- Testing of Equipment Interlocks
- Speed Tests
What is PQ or Performance Qualification?
Performance Qualification confirms that the equipment and systems meet the users’ needs and is fit for intended use as defined in the user requirements specification (URS). It is the final step in equipment qualification.
It is much like Operational Qualification, as it tests the operational requirements of the equipment, but in this case, the equipment will contain a load or process medium.
In other words, you test the equipment while it’s being subject to “real-world” conditions – the conditions that the equipment will be subject to during batch production.
This phase is hugely important as it combines the workings, forces and energy of the individual components of the equipment into one harmonious system. In doing so, this phase of qualification can identify faults such as:
- Excessive vibration or noise caused by the combination of 2 or more vibrating components leading to resonance
- Excessive heat caused by a driving belt
- Process media backflow caused by incorrectly sized pipework
- Pressure differentials caused by valves, other equipment or process media backflow
- Faults (like those above) combining to cause larger problems in operation
- Inconsistency of operation
- Problems with types of operations (such as mixing, blending, and granulation)
- Chemical impurities
- Incorrect particle sizes
- Issues with content uniformity
Performance Qualification must not be confused with Process Validation (PV) (verification that good product is made) or with cleaning validation and analytical methods.
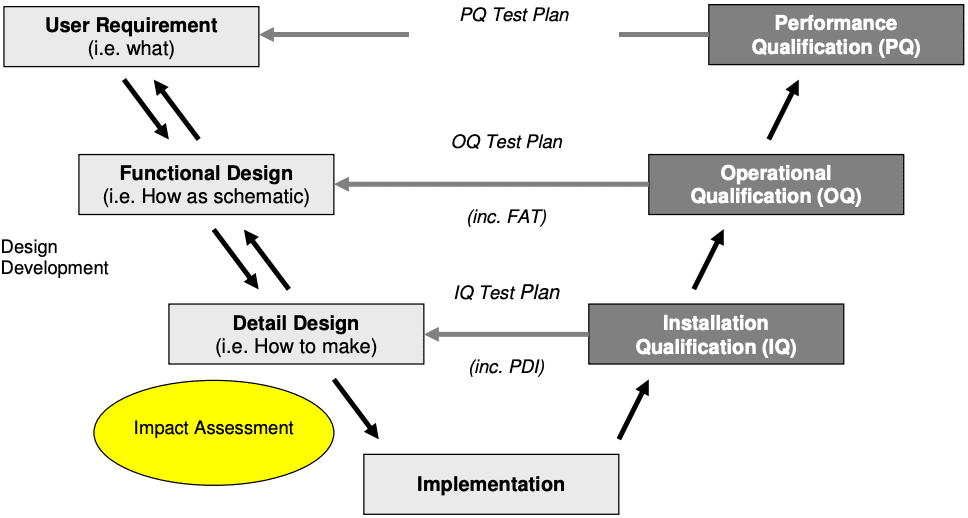
What is the V-Model for Direct Impact Systems?
The V-model is a method used to visualize and compare the relationship between the user requirements, functional design and detailed design to the installation qualification (IQ), operational qualification (OQ) and performance qualification (PQ) performed on them (see diagram below).
To implement, you start at the top left with user requirements, work down the left hand side of the V, and then up the right hand side, ending at PQ.
The left-hand side of the V represents what you want the system to do and how you’ll achieve that . The right-hand side of the V represents how you test the system to confirm that the system is fit-for-purpose (i.e. will make safe medicines for patients).

In pharmaceutical manufacturing, most companies and organisations follow the ISPE’s V-Model (ISPE Baseline Guide Volume-5) to validate their systems as it meets the requirements of the industry regulators such as the Food and Drug Administration (FDA) or the European Medicines Association (EMA).
What is an Equipment Qualification Protocol?
An Equipment Qualification Protocol is a written plan stating how the qualification process will be conducted. It includes a component-level impact assessment, the steps to perform IQ OQ and PQ, test parameters, product characteristics, production equipment and decision points on what constitutes an acceptable result.
It details factors such as:
- Test scripts and methods – telling you the steps involved in conducting a test
- Test parameters and acceptance criteria – defining acceptable test results
- Product characteristics – showing what your system is looking to achieve/produce
- Production equipment – detailing the equipment necessary
- Final approval – documenting that the validation process has been successfully carried out
This flowchart provides a broad overview of the process used to gain approval to execute the protocol and shows the expected time and responsibilities for developing it.
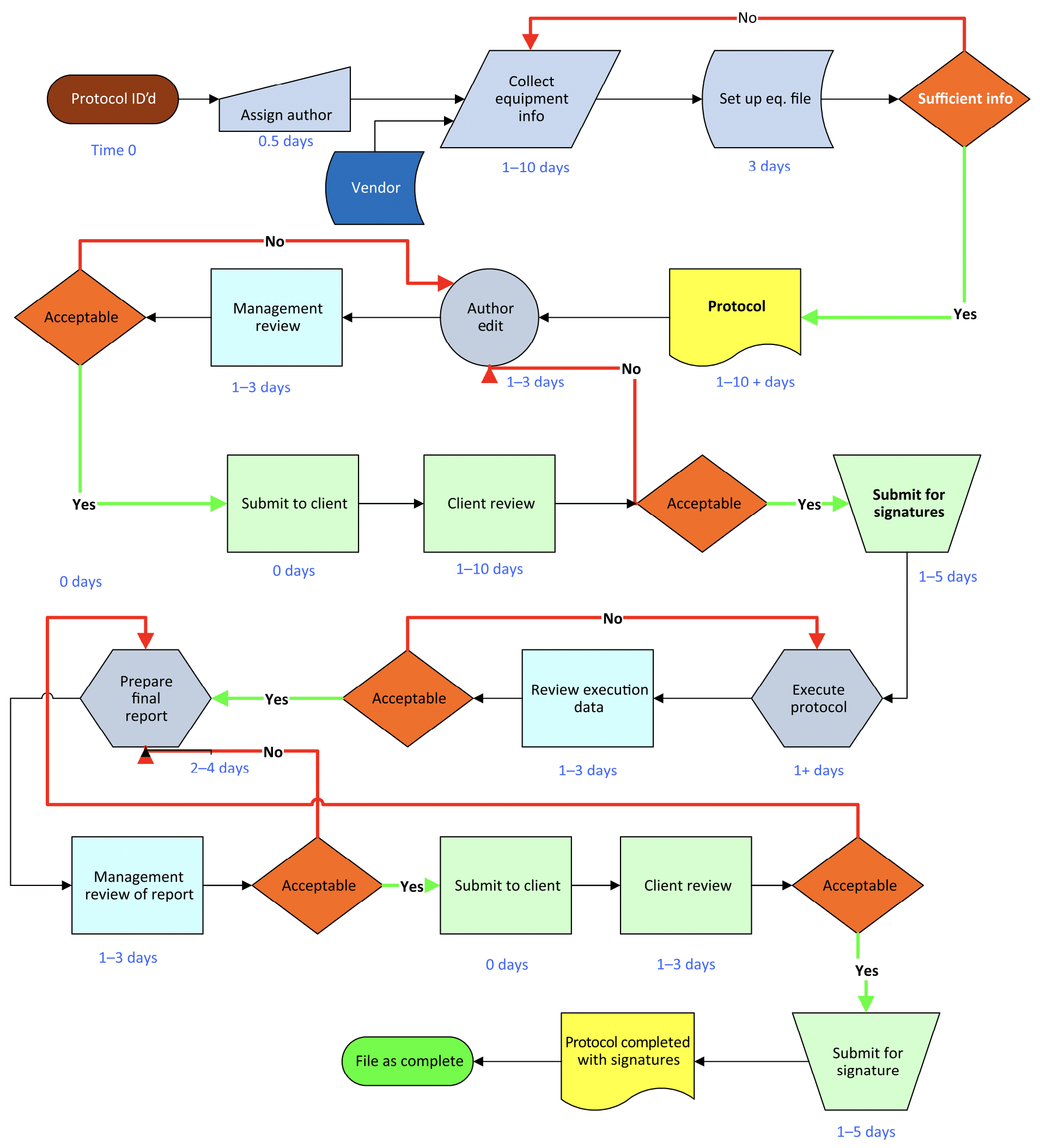
Image: Equipment Qualification in the Pharmaceutical Industry by Steven Ostrove
Not every protocol needs to follow this specific path or the indicated timeline (often depending on whether the protocol is prepared in-house or by an outside firm) but there are some general approaches and steps that have proven successful over the years that you’re likely to see included.
It can be useful to think of a qualification protocol as a highly detailed checklist with 2 steps.
Step 1: Write and develop the protocol
This is a detailed document and contains the elements outlined above. This step is usually performed by a senior validation engineer, CQV or C&Q specialist and requires a lot of experience and detailed knowledge of the process.
Pre-preparation checklist.
1) Perform a systems-level impact assessment to make sure you are only qualifying systems that have a direct or indirect impact on product quality and patient safety.
2) Get a thorough understanding of the unit’s function. You must understand the unit’s function and its intended use. If a unit has multiple functions, only those being utilized for the current operation need to be qualified. This includes ensuring that unqualified functions do not interfere with the operation of the qualified ones.
3) Inquire from the users (if possible) about the system’s expected specific function.
4) Gather necessary documents such as equipment manuals, URS, functional specifications, design specifications, and standard operating procedures (SOPs).
Documents needed for IQ OQ PQ preparation
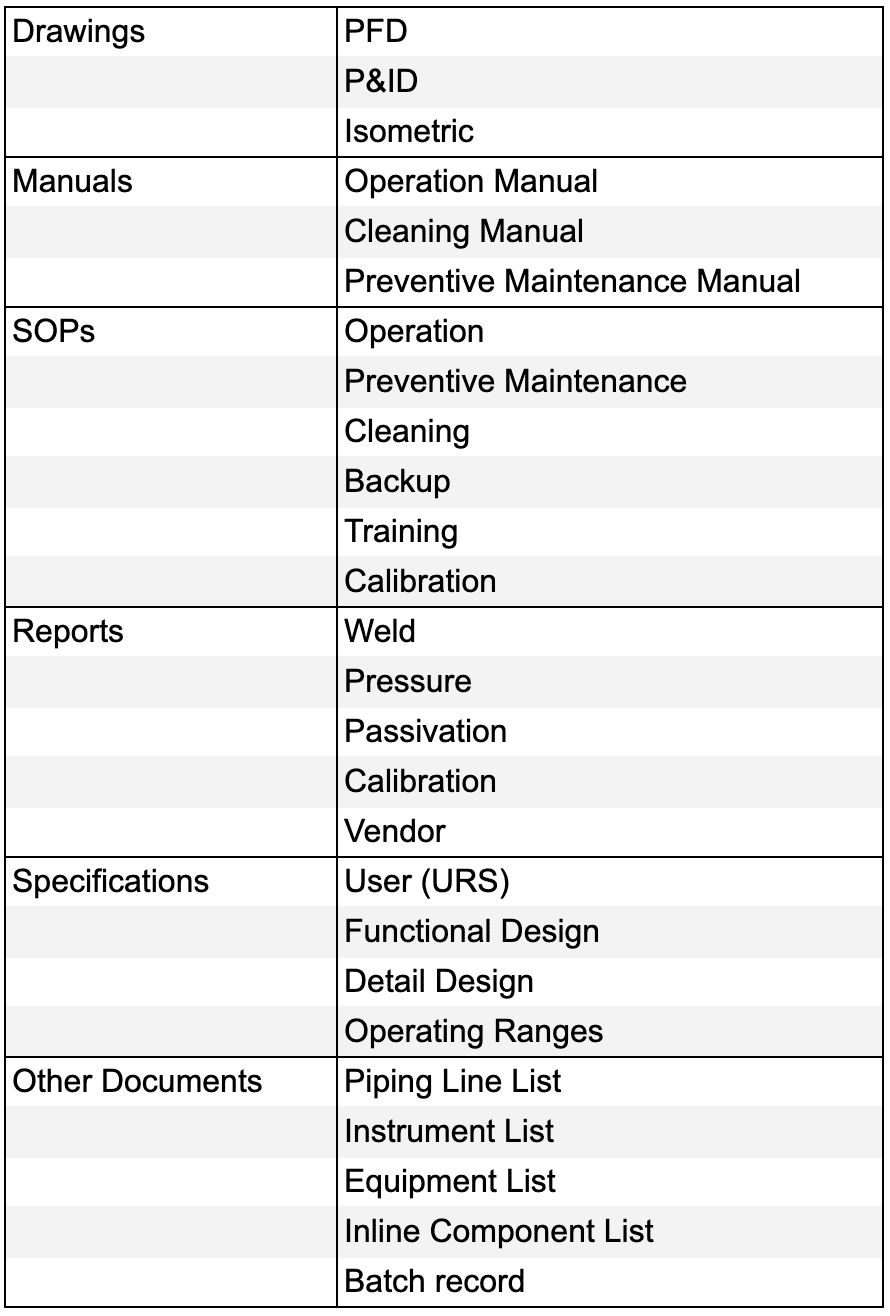
5) Review the Process Flow Diagram (PFD) to grasp the overall process flow.
6) Review the Piping and Instrumentation Diagram (P&ID) to confirm the system boundaries. Each qualification protocol must represent a stand-alone system capable of operating independently. An example of such a system is a Clean In Place System, which, despite having many internal components crucial for its operation, presents itself as a complete unit. Below is an example of the boundaries drawn in yellow around a Clean-In-Place system.
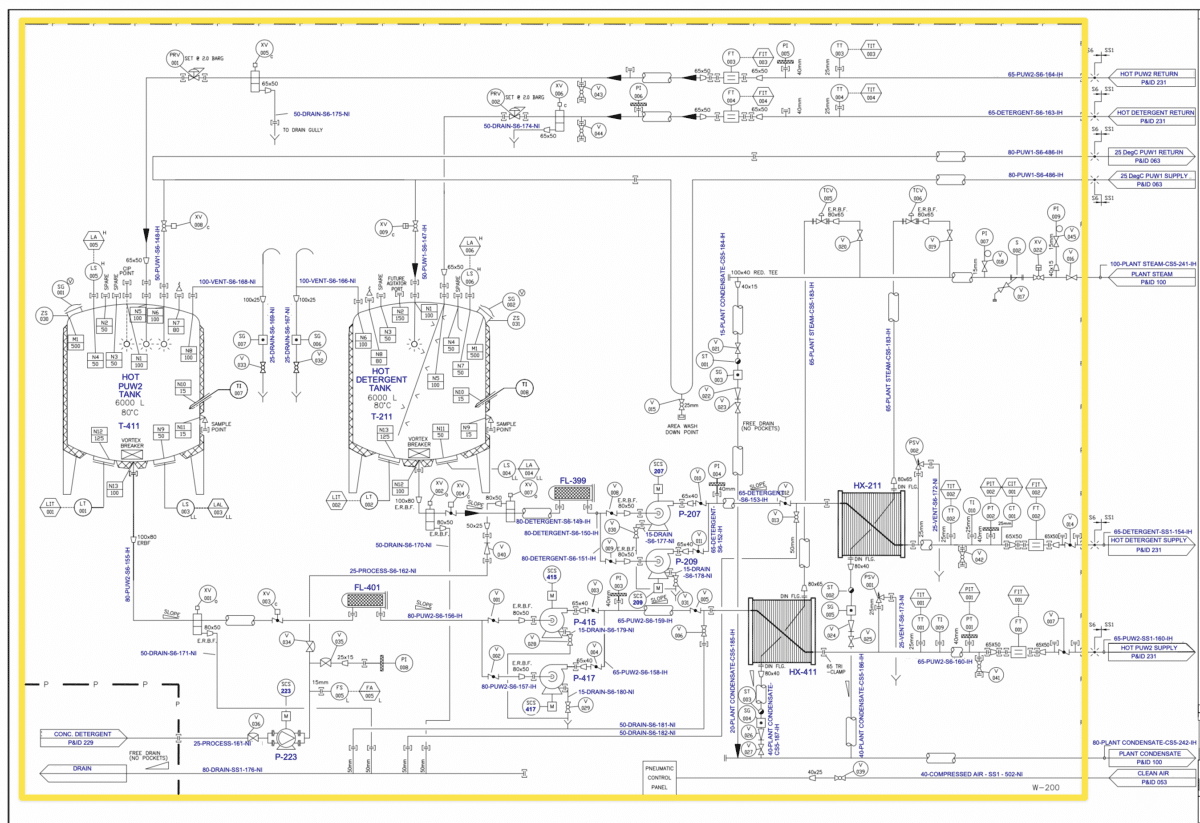
7) Review the commissioning process for the unit to see if any component or design changes have been made.
8) Review the Factory Acceptance Tests (FAT), Site Acceptance Tests (SAT), and commissioning tests conducted, along with the history of the process (e.g., similar products manufactured using comparable equipment).
9) Determine if other machines of a similar type are in use to see if that knowledge can be used in the development of the protocol.
10) Inquire if there are any existing protocols or products similar to the one to be manufactured in the unit, which currently utilize similar equipment.
11) Thoroughly review current regulatory guidelines and current warning letters. Understanding the FDA or other regulatory agencies’ expectations is essential for crafting a clear and concise protocol.
Preparing the Protocol
- Only qualify critical systems and critical components: Perform a component impact assessment to develop a critical components list and only qualify those critical systems and those components within the system that are essential for the unit’s operation or have direct impact or contact with the product. Non-essential elements, such as lightning or steam supply, etc do not require qualification.
- Prepare protocol: Prepare the protocol with predetermined acceptance criteria. You need to have a planned approach to qualification, ensuring that all testing aligns with user requirements specifications (URS) rather than design specifications alone.
- Format: Protocols need to have a specific defined structure or format. However, this format is not as important as its content. Once a format has been established for a company or consultancy, try to maintain this format for future protocols.
Step 2: How to execute an IQ OQ or PQ protocol
This is where you take the documents (paper or electronic) out on-site and execute each of the IQ OQ or PQ protocols. You’ll be leaving your desk and going out onto the factory floor with the checklist in hand. You’ll then use it to test and confirm everything is correctly installed, properly configured, and works as intended under load.
Pre-execution checklist
- Review the protocol and confirm what is to be tested and how it is to be performed.
- Make sure you have the current (correct) version of the protocols that are to be executed and review them before starting the execution.
- Check that the protocols have been fully approved (by QA, Engineering, Maintenance, Calibration, etc) before starting the execution.
- Check that the equipment is ready and available for the current protocol execution (e.g. it may have been approved for use in or for another product or situation).
- Review safety procedures.
- Notify owners of scheduled validation work.
- Collect necessary test instruments, check they’re all calibrated and that their calibration certificates are available for attachment to the protocol.
- Notify any specialists you need to assist with testing (e.g. operators, electricians, fitters).
- Obtain relevant SOPs.
- Make a COPY of the protocol(s) to be executed and ensure that all pages are accounted for.
Completing the execution
- Do not make assumptions.
- Make sure that all piping is clearly identified and tagged.
- Make sure that utility connections are correct and tagged.
- All data/entries should be made on the protocol page. Additional pages may be added if necessary, with a unique number.
- All entries should be neat and legible to others.
- Don’t leave any blank lines or spaces.
- Make sure to follow Good Documentation Practices.
- The IQ protocol must be signed before the OQ can be signed.
- The OQ protocol must be signed before the PQ can be signed.
- All deviations must be closed before signing any protocol as complete.
- All data are entered and either initialled or signed and dated at the point of execution. -No pre or post-dating is allowed.
Always Remember!
- The goal is to make safe medicines at an affordable cost – you must balance these objectives. There can be a tendency, especially amongst novice C&Q technicians and engineers to qualify all components in a system. However, the qualification process is enormously time-consuming and expensive so this approach drives up the cost of qualifying and validating the project and subsequently the final price of medicine way higher than necessary (which makes it unaffordable to less well-off patients). The solution is to use system impact assessments, component impact assessments and risk management tools in a scientifically robust manner to support your decisions about what to validate to avoid over-qualifying.
- Use commissioning data wherever possible to reduce testing duplication. The quality assurance department will need to approve.
- There is no single right answer or a “perfect” approach to validating a project. In fact, there are always multiple right answers and approaches. The key point is that you must be able to explain your rationale to an FDA or EMA auditor or supervisor. As long as your rationale is sound and logical so that even if someone disagrees with you, they can understand the decision, you won’t be penalised (even if you are asked to change it).
Next Steps
If you need to learn how to populate an Equipment Validation Protocol, check out our Equipment Validation (IQ OQ PQ) Training Course – For Starter Validation, CQV and C&Q Roles where we walk you through this entire qualification process step by step.
About the Authors
Gerry Creaner
President
Senior Lecturer with GetReskilled
Gerry Creaner has over 30-years of experience in the Life Sciences Manufacturing industry across a range of technical, managerial and business roles. He established a very successful engineering consultancy prior to founding GetReskilled, an online education and learning business, with offices in Singapore, Ireland and Boston (USA), focussed on the manufacture of safe and effective medicines for the public.
He is also a founding Director of two Singapore based philanthropic organizations, the Farmleigh Fellowship and the Singapore-Ireland Fund, both of which deepen the well established and historical Singapore – Ireland relationship and deliver long-term benefits to both countries.
Gerry has an undergraduate degree in Chemical Engineering (UCD, 1980) and an MSc (Management) from Trinity College Dublin (2003) and is currently doing research for his Ph.D.
Donagh Fitzgerald
Head of Marketing & Product Development
Mechanical/Production Engineer
Donagh looks after the marketing and product development including the training and pedagogical elements of our programs and makes sure that all GetReskilled’s users can have a great online learning experience. Donagh has lived and worked in many countries including Ireland, America, the UK, Singapore, Hong Kong and Japan. Donagh has also served as the Program Manager for the Farmleigh Fellowship based out of Singapore.
Donagh holds Degrees in Production Engineering and Mechanical Engineering from South East Technological University, Ireland.

